��Ŀ����
��12�֣���ѧʵ����ȤС����ʵ���ҽ��������������е�ȼ��ʵ�顣
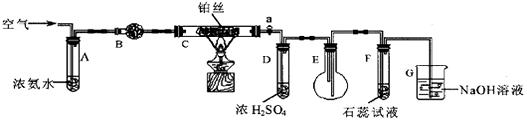
��һ���������ͼ��ʾװ��I�Ʊ����ս����﴿���������Ա�����ʵ�飻
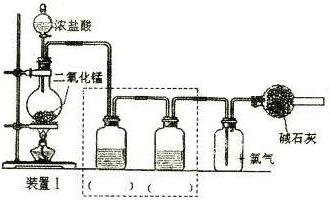
��ƿ�з�����Ӧ�����ӷ���ʽΪ ��
�����ʵ��Ŀ�Ľ�װ��I�����߿���װ�ò�����������������ע�������Լ���
����������ͼ��ʾװ�â��У����Թ����ȼ���������ۣ��ٵ�������CaSO4��Һ��������һ����ϡ���ᣬ���Ͻ������۲쵽�д������ݲ�����
�ټ����ȡ����װ�������ԵIJ���������
�ڵ���ȼ�����������ʢ�������ļ���ƿ�У��۲쵽��������
������������ֹͣȼ�պ�ȡ�����ܣ�ͬѧ�ǽ����˷���̽��ʵ�顣A��ͬѧ��������������Թ��л��н϶����ʣ�࣬ͬѧ�ǽ���������ͨ���Թ��У����ֹ���ȫ����ʧ����δ�۲쵽�����ݲ���������Ϳ���ԭ�������ӷ���ʽ��ʾ���� ��B��ͬѧ����ƿ��ע������ˮ��������������������Na2SO3��Һ��������Һ��û�г��ֻ�ɫ��������������Һ�е���ϡ�����ữ���Ȼ�����Һ�������˰�ɫ������[��֪Na2S2O3+H2SO4=Na2SO4+S��+SO2��+H2O]��ͬѧ�Ƿ��������ڼ���ƿ�л�����������������Ե�ʡ����Խ���ʵ��������������ӷ���ʽ�ǣ�
��
��һ���������ͼ��ʾװ��I�Ʊ����ս����﴿���������Ա�����ʵ�飻

��ƿ�з�����Ӧ�����ӷ���ʽΪ ��
�����ʵ��Ŀ�Ľ�װ��I�����߿���װ�ò�����������������ע�������Լ���

����������ͼ��ʾװ�â��У����Թ����ȼ���������ۣ��ٵ�������CaSO4��Һ��������һ����ϡ���ᣬ���Ͻ������۲쵽�д������ݲ�����
�ټ����ȡ����װ�������ԵIJ���������
�ڵ���ȼ�����������ʢ�������ļ���ƿ�У��۲쵽��������
������������ֹͣȼ�պ�ȡ�����ܣ�ͬѧ�ǽ����˷���̽��ʵ�顣A��ͬѧ��������������Թ��л��н϶����ʣ�࣬ͬѧ�ǽ���������ͨ���Թ��У����ֹ���ȫ����ʧ����δ�۲쵽�����ݲ���������Ϳ���ԭ�������ӷ���ʽ��ʾ���� ��B��ͬѧ����ƿ��ע������ˮ��������������������Na2SO3��Һ��������Һ��û�г��ֻ�ɫ��������������Һ�е���ϡ�����ữ���Ȼ�����Һ�������˰�ɫ������[��֪Na2S2O3+H2SO4=Na2SO4+S��+SO2��+H2O]��ͬѧ�Ƿ��������ڼ���ƿ�л�����������������Ե�ʡ����Խ���ʵ��������������ӷ���ʽ�ǣ�
��
��MnO2+4H++2Cl- Mn2++Cl2��+2H2O��2�֣�
 ��2�֣���
��2�֣���
��������ĩ�˲���ˮ���У�������ס�Թܣ��ڵ�����ĩ�˻������ݲ������ɿ��ֺ�����ĩ����һ��ˮ����������֤����װ�õ����������ã�1�֣�������������ȼ�գ�������ɫ���棬ƿ�ڳ��ְ�����1�֣�
��Cl2+2Fe2+= Cl-+2Fe3+ 2Fe3++Fe= 3Fe2+ 2Fe3++Cu = Cu2++ 2Fe2+
3Cl2+2Fe=" 2Fe" Cl3��4�֣�
4Cl2+S2O32-+5H2O=2SO42-+8Cl-+10H+ Ba2++SO42-=BaSO4����2�֣�
 ��2�֣���
��2�֣�����������ĩ�˲���ˮ���У�������ס�Թܣ��ڵ�����ĩ�˻������ݲ������ɿ��ֺ�����ĩ����һ��ˮ����������֤����װ�õ����������ã�1�֣�������������ȼ�գ�������ɫ���棬ƿ�ڳ��ְ�����1�֣�
��Cl2+2Fe2+= Cl-+2Fe3+ 2Fe3++Fe= 3Fe2+ 2Fe3++Cu = Cu2++ 2Fe2+
3Cl2+2Fe=" 2Fe" Cl3��4�֣�
4Cl2+S2O32-+5H2O=2SO42-+8Cl-+10H+ Ba2++SO42-=BaSO4����2�֣�
��

��ϰ��ϵ�д�
 ������ҵ����ν�����������ϵ�д�
������ҵ����ν�����������ϵ�д�
�����Ŀ
 ��Һ��ֱ�����ٲ���������Ȼ�����û����ת
��Һ��ֱ�����ٲ���������Ȼ�����û����ת �Ƶ���������������ȫ����Ϊ��ɫ��
�Ƶ���������������ȫ����Ϊ��ɫ�� 2g
2g
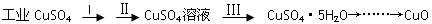



 2NH3(g)����H=-92.2kJ��mol-1��
2NH3(g)����H=-92.2kJ��mol-1��