��Ŀ����
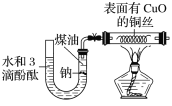
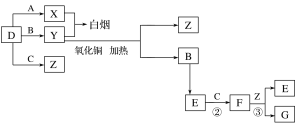
����Ŀ����ͼ��ʾ����֪���л���A����Է���������28�����IJ����Ǻ���һ������ʯ�ͻ���ˮƽ�ı�־;��2CH3CHO+O2![]() CH3COOH����B��D�����ճ�����ʳƷ�г������л����E�Ǿ���Ũ����ζ����������ˮ����״Һ�壻��F��һ�ָ߾����������������ʳ�ﱣ��Ĥ��
CH3COOH����B��D�����ճ�����ʳƷ�г������л����E�Ǿ���Ũ����ζ����������ˮ����״Һ�壻��F��һ�ָ߾����������������ʳ�ﱣ��Ĥ��
�ش��������⣺
(1)д��A�Ľṹ��ʽ_________��D�Ľṹ��ʽ_________
(2)д�����з�Ӧ�ķ�Ӧ���ͣ���_________����_________
(3)д����Ӧ�ܵĻ�ѧ����ʽ��_________
���𰸡�CH2=CH2 CH3COOH ������Ӧ ������Ӧ(ȡ����Ӧ) CH3CH2OH+CH3COOH![]() CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O
��������
�л���A����Է���������28�����IJ����Ǻ���һ������ʯ�ͻ���ˮƽ�ı�־����AΪ��ϩ(CH2=CH2)����ϩ��ˮ�ڴ��������·����ӳɷ�Ӧ���ɵ�BΪCH3CH2OH���Ҵ������������ɵ�CΪCH3CHO����ȩ�ٱ��������ɵ�DΪCH3COOH��������Ҵ�����������Ӧ���ɵ�EΪCH3COOCH2CH3����ϩ�ڴ��������·����Ӿ۷�Ӧ���ɵ�FΪ����ϩ��
(1)AΪ��ϩ���ṹ��ʽΪCH2=CH2��DΪ���ᣬ�ṹ��ʽΪCH3COOH��
(2)��Ӧ��Ϊ�Ҵ��Ĵ�����������������Ӧ����Ӧ��Ϊ�Ҵ��������������Ӧ����Ӧ����Ϊ������Ӧ(��ȡ����Ӧ)��
(3)��Ӧ�ܵĻ�ѧ����ʽΪ��CH3CH2OH+CH3COOH![]() CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��