��Ŀ����
��Ԫ�صĻ���������࣬����Ҳ������ͬ��
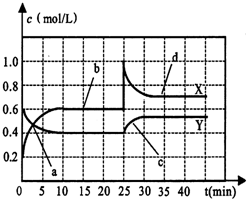
��1��NO2�н�ǿ�������ԣ��ܽ�SO2��������SO3����������ԭΪNO����֪��������Ӧ�����������仯��ͼ��ʾ��

��NO2����SO2���Ȼ�ѧ����ʽΪ_________________________________��
��2����2L�ܱ������з���1mol��������һ���¶Ƚ������·�Ӧ��
2NH3(g) N2��g��+3H2��g������Ӧʱ��(t)��������������ѹǿ(p)�����ݼ��±�
N2��g��+3H2��g������Ӧʱ��(t)��������������ѹǿ(p)�����ݼ��±�
ʱ��t/min | 0 | 1 | 2 | 3 | 4 | 5 |
��ѹǿp 100 kPa | 5 | 5.6 | 6.4 | 6.8 | 7 | 7 |
��ƽ��ʱ������ת����Ϊ___________��
��3���£�N2H4���ֳ���������һ�ֿ�ȼ�Ե�Һ�壬���������ȼ�ϡ��ڿ�������ȫȼ�����ɵ���������Ӧת��0.2mol����ʱ�����������ڱ�״���µ����Ϊ______________����������ˮ���Է����백ˮ���Ƶĵ��룬��д��������ˮ��Һ�еĵ��뷽��ʽ��
__________________��дһ�����ɣ���
��4��NH4+����Һ���ܷ���ˮ�ⷴӦ����25��ʱ��0.1mol/L�Ȼ����Һ��ˮ�������������Ũ��Ϊ1��10-5 mol/L�����ڸ��¶��´���Һ�а�ˮ�ĵ���ƽ�ⳣ��Kb��NH3��H2O��=__________________��
��1��NO2��g��+SO2��g��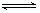 SO3��g��+NO��g�� ��H=��41.8kJ/mol��2����
SO3��g��+NO��g�� ��H=��41.8kJ/mol��2����
��2��40%��2����
��3�� 1.12L��2������N2H4��H2O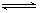 N2H5����OH�� ��2����
N2H5����OH�� ��2����
��4�� Kb��NH3��H2O����1��10-5mol/L��2����
��������
�����������1������ͼ1�ɵã�2SO2(g)+O2(g) 2SO3(g)? ��H=��196.6kJ?mol?1������ͼ2�ɵã�2NO(g)+O2(g)=2NO2(g)? ��H=��113.0kJ?mol?1�����ݸ�˹���ɿɵ�NO2��g��+SO2��g��
2SO3(g)? ��H=��196.6kJ?mol?1������ͼ2�ɵã�2NO(g)+O2(g)=2NO2(g)? ��H=��113.0kJ?mol?1�����ݸ�˹���ɿɵ�NO2��g��+SO2��g��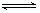 SO3��g��+NO��g�� ��H=1/2��H1��1/2��H2=��41.8kJ?mol?1
SO3��g��+NO��g�� ��H=1/2��H1��1/2��H2=��41.8kJ?mol?1
��2�������ѹǿ֮�ȵ������ʵ���֮�ȣ���������ʽ���м��㣺�谱����ת����Ϊx
???????????????????? 2NH3(g) N2��g��+3H2��g��
N2��g��+3H2��g��
��ʼ���ʵ�����mol��?? 1???????? 0??????? 0
ת�����ʵ�����mol��?? x???????? x/2????? 3/2x
ƽ�����ʵ�����mol��? 1-x??????? x/2?????? 3/2x
1����1+x��=5:7�����x=40%
��3��N2H4ת��ΪN2��NԪ���� -2��������0�ۣ����Ե���ת��Ϊ��N2H4 ~ N2 ~ 4e?��n��N2��=1/4n��e?��=0.05mol�����ڱ�״���µ����Ϊ1.12L����������ˮ���Է����백ˮ���Ƶĵ��룬N2H4��H+�γ���λ�������Ե��뷽��ʽΪ��N2H4��H2O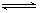 N2H5����OH��
N2H5����OH��
��4��c(OH?)=Kw/c(H+)=10-9mol?L?1��c(NH3?H2O)=c(H+)=10-5mol?L?1������Kb��NH3��H2O��=c(NH4+)?c(OH?)/c(NH3?H2O)=0.1mol/L��10-9mol?L?1/10-5mol?L?1=1��10-5mol/L
���㣺���⿼���Ȼ�ѧ����ʽ����д����ѧƽ���ƽ�ⳣ���ļ��㡢���뷽��ʽ����д��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д� ����������Ԫ��A��B��C��D��E��ԭ������������������ԭ�Ӻ���ĵ��Ӳ���֮��Ϊ10��BԪ�صĻ���������࣬��Ŀ�Ӵ�C��D����Ԫ���γɵĵ����ǿ����к����������ʣ�D��E��Ԫ�ؿ����������ֲ�ͬ�����ӻ����
����������Ԫ��A��B��C��D��E��ԭ������������������ԭ�Ӻ���ĵ��Ӳ���֮��Ϊ10��BԪ�صĻ���������࣬��Ŀ�Ӵ�C��D����Ԫ���γɵĵ����ǿ����к����������ʣ�D��E��Ԫ�ؿ����������ֲ�ͬ�����ӻ���� NH3?H2O+H+
NH3?H2O+H+ 2NO2
2NO2