��Ŀ����
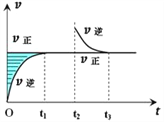
����Ŀ��һ���¶��£������Ϊ10L���ܱ������У�3molX��1molY����Ӧ��2X��g��+Y��g��![]() Z��g������2min�ﵽƽ�⣬����0.6molZ������˵����ȷ����
Z��g������2min�ﵽƽ�⣬����0.6molZ������˵����ȷ����
A. ��XŨ�ȱ仯��ʾ�ķ�Ӧ����Ϊ0.01mol/��L��s��
B. �����������Ϊ20L��Z��ƽ��Ũ��Ϊԭ����1/2
C. ������ѹǿ��������Y��ת���ʼ�С
D. �������¶ȣ�X���������������÷�Ӧ����H<0
���𰸡�D
��������
A�����ݻ�ѧ��Ӧ���ʹ�ʽ����Z�����ʣ������ݻ�ѧ��Ӧ����֮�ȵ��ڻ�ѧ������֮�ȼ���X���ʣ�
B���÷�Ӧ��һ�����������С�ķ�Ӧ����ϵ��ѹǿ��С��ƽ�����淴Ӧ�����ƶ���
C���÷�Ӧ��һ�����������С�ķ�Ӧ������ѹǿ��ƽ��������Ӧ�����ƶ���
D�������¶ȣ�ƽ�������ȷ����ƶ���
A���2min�ﵽƽ�⣬����0.6molZ��Z��Ũ�ȱ仯��Ϊ0.06mol/L��Z�ķ�Ӧ����v��Z��Ϊ0.06mol/L��120s��0.0005mol/��Ls�������ݻ�ѧ��Ӧ����֮�ȵ��ڻ�ѧ������֮�ȣ��ɷ���ʽ��֪v��X��=2v��Z��=2��0.0005mol/��Ls��=0.00lmol/��Ls������A����
B������������Ϊ20L����ƽ�ⲻ�ƶ���Z��Ũ�ȱ�Ϊԭ����1/2���÷�Ӧ��һ�����������С�ķ�Ӧ����ϵ��ѹǿ��С��ƽ�����淴Ӧ�����ƶ���Z��ƽ��Ũ��С��ԭ����1/2����B����
C��÷�Ӧ��һ�����������С�ķ�Ӧ������ѹǿ��ƽ��������Ӧ�����ƶ�����Ӧ��Y��ת��������C����
D������¶ȣ�X�������������˵�������¶�ƽ�����淴Ӧ�����ƶ��������¶ȣ�ƽ�������ȷ����ƶ���������Ӧ�ġ�H��0����D��ȷ��
��ѡD��

 �żӾ���ϵ�д�
�żӾ���ϵ�д�����Ŀ����֪ ��2SO2(g)+ O2(g)![]() 2SO3(g) ��H= ��QkJ/mol�����¶�һ�����ݻ�Ϊ2L���ܱ������зֱ������������ʵ�飺������ú��ʵ��1��Ӧ�ų�������ΪQ1kJ��ʵ��2û�������仯��������˵���в���ȷ���ǣ� ��
2SO3(g) ��H= ��QkJ/mol�����¶�һ�����ݻ�Ϊ2L���ܱ������зֱ������������ʵ�飺������ú��ʵ��1��Ӧ�ų�������ΪQ1kJ��ʵ��2û�������仯��������˵���в���ȷ���ǣ� ��
ʵ���� | SO2(g) | O2(g) | SO3(g) |
1. | 2mol | 1mol | 0mol |
2. | 1mol | 0.5mol | 1mol |
3. | 1mol | 0.8mol | 1.4mol |
A. ʵ��1�ų�������ֵΪQ1=0.5Q B. �������µ�ƽ�ⳣ��Ϊ4
C. ʵ��1��O2��ת����Ϊ50% D. ʵ��3��Ӧ�ų�����
����Ŀ������ͼװ�ý���ʵ�飬�����ƶ���ȷ����(����)
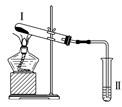
ѡ�� | �����Լ� | �����Լ������� | �ƶ� |
A | �Ȼ�� | ��̪��Һ�����ɫ | �Ȼ���ȶ� |
B | �������� | Ʒ����Һ��ɫ | FeSO4�ֽ�����FeO��SO2 |
C | Ϳ��ʯ���͵����Ƭ | ���Ը��������Һ��ɫ | ʯ���ͷ����˻�ѧ�仯 |
D | ������ˮ���� | ����ˮð�� | ������ˮ���������˷�Ӧ |
A. A B. B C. C D. D