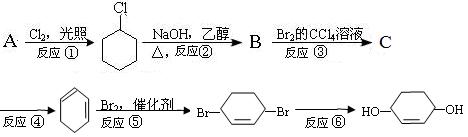
��Ŀ����
 ij�������Ļ�����A������Է�������Ϊ104��̼����������Ϊ92.3����
ij�������Ļ�����A������Է�������Ϊ104��̼����������Ϊ92.3���� ��1��A�ķ���ʽΪ
��1��A�ķ���ʽΪ  ��A�����������ͬһƽ���ԭ����Ϊ ��
��A�����������ͬһƽ���ԭ����Ϊ �� ��2��һ�������£�A��������Ӧ���õ��Ļ�������̼����������Ϊ85.7��
��2��һ�������£�A��������Ӧ���õ��Ļ�������̼����������Ϊ85.7�� ��д���˻�����Ľṹ��ʽ ��
��д���˻�����Ľṹ��ʽ �� ��3����֪��
��3����֪��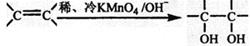 ����д��A��ϡ�����KMnO4��Һ�ڼ��������·�Ӧ����Ľṹ��ʽ ��
����д��A��ϡ�����KMnO4��Һ�ڼ��������·�Ӧ����Ľṹ��ʽ �� ��4���������Ϲ����ԳƼӳ�ʱ��ͨ������ӵ������IJ�����̼ԭ��һ�ࡱ,д��A��HBr��Ӧ��Ҫ����B�Ľṹ��ʽ ��������B�ж���ͬ���칹�壬д��������������������ͬ���칹��Ľṹ��ʽ�� ��
��4���������Ϲ����ԳƼӳ�ʱ��ͨ������ӵ������IJ�����̼ԭ��һ�ࡱ,д��A��HBr��Ӧ��Ҫ����B�Ľṹ��ʽ ��������B�ж���ͬ���칹�壬д��������������������ͬ���칹��Ľṹ��ʽ�� ���ٺ����� �ڱ�����ֻ�����ֲ�ͬ��ѧ��������ԭ�ӡ�

��

��ϰ��ϵ�д�
 ���Ǽ���С����ϵ�д�
���Ǽ���С����ϵ�д� �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д�
�����Ŀ
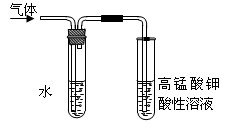
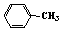 )����b��һ�ȴ����a��b����ֵ�ֱ�Ϊ
)����b��һ�ȴ����a��b����ֵ�ֱ�Ϊ 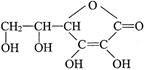 �����ķ���ʽ��______________���������ܷ��λ�Ѫ�����ֳ�Ϊ_________________����ά����C��Һ�е�����ɫʯ����Һ����Һ��ɫ��죬˵��ά����C��Һ����__________�ԣ���ά����C��Һ�е���������ɫ�ĺ��е��۵ĵ�ˮ���ɹ۲쵽��������______________��˵��ά����C����_______________�ԡ�
�����ķ���ʽ��______________���������ܷ��λ�Ѫ�����ֳ�Ϊ_________________����ά����C��Һ�е�����ɫʯ����Һ����Һ��ɫ��죬˵��ά����C��Һ����__________�ԣ���ά����C��Һ�е���������ɫ�ĺ��е��۵ĵ�ˮ���ɹ۲쵽��������______________��˵��ά����C����_______________�ԡ�
 HBr+NaHSO4 ��
HBr+NaHSO4 �� R-Br+H2O ��
R-Br+H2O ��