��Ŀ����
ij��ѧС����ʵ��������CaSO4��NH3��CO2�Ʊ�(NH4)2SO4���乤���������¡�
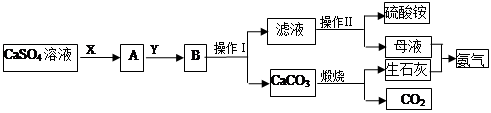
�ش��������⣺
��1�������������Ϊ_________��������һϵ�в�����������Ũ����________�����ˡ�
��2��ʵ����������̼���ʱ��ʢ��̼������õ�������________(������)��
��3��X����Ϊ____(�ѧʽ����ͬ)��Y����Ϊ____����ѭ�����õ����ʵ���_____��
��4��Ҫ�ⶨ���Ƶõ�����林��ȣ�ȡ10.0g��Ʒ����ȫ����ˮ������Һ�еμӹ������Ȼ�����Һ�����ˡ�ϴ�ӡ������������������Ϊ16.31g��Ϊ���������������Ȼ�����Һ�Ƿ������õ��Լ���_______�����Ƶ�����淋Ĵ���Ϊ________��
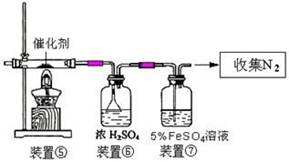
��5������װ�ò�������ʵ�����ư�������__________(�����)��
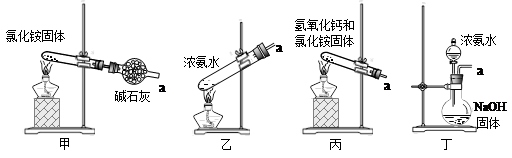
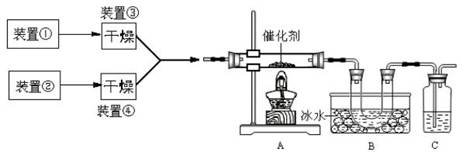
ѡ��������ȡװ�ú���������װ���ռ�����İ���������ȡ�������Һ�����ӵ�˳��(�ýӿ������ĸ��ʾ)�ǣ�a��____��____��____��____��_____��____��_____��

����װ����CCl4��������___________________��
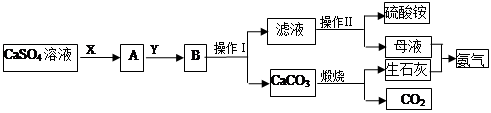
�ش��������⣺
��1�������������Ϊ_________��������һϵ�в�����������Ũ����________�����ˡ�
��2��ʵ����������̼���ʱ��ʢ��̼������õ�������________(������)��
��3��X����Ϊ____(�ѧʽ����ͬ)��Y����Ϊ____����ѭ�����õ����ʵ���_____��
��4��Ҫ�ⶨ���Ƶõ�����林��ȣ�ȡ10.0g��Ʒ����ȫ����ˮ������Һ�еμӹ������Ȼ�����Һ�����ˡ�ϴ�ӡ������������������Ϊ16.31g��Ϊ���������������Ȼ�����Һ�Ƿ������õ��Լ���_______�����Ƶ�����淋Ĵ���Ϊ________��
��5������װ�ò�������ʵ�����ư�������__________(�����)��

ѡ��������ȡװ�ú���������װ���ռ�����İ���������ȡ�������Һ�����ӵ�˳��(�ýӿ������ĸ��ʾ)�ǣ�a��____��____��____��____��_____��____��_____��

����װ����CCl4��������___________________��
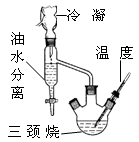
(1)����(1��)����ȴ�ᾧ(2�֣���һ�����1��)
(2)����(1��)
(3)NH3��CO2�� NH3��CO2(ÿ�����ʾ�Ϊ1�֣����һ����һ�ֿ�1�֣���һ�ֵ���1��)
(4)�Ȼ�����Һ(2�֣�)��92.4%(3��)
(5)��(2��)��de��gf��cb��h(2��)��������(1��)
(2)����(1��)
(3)NH3��CO2�� NH3��CO2(ÿ�����ʾ�Ϊ1�֣����һ����һ�ֿ�1�֣���һ�ֵ���1��)
(4)�Ȼ�����Һ(2�֣�)��92.4%(3��)
(5)��(2��)��de��gf��cb��h(2��)��������(1��)
�����������1����������I�õ�CaCO3�������Һ�����Բ���IΪ���ˣ�����淋��ܽ�����¶ȵĽ��Ͷ���С����������Ũ���������ȴ�ᾧ��
��2��ʵ�����и������չ��������Ϊ������
��3��������Ŀ��Ϣ��ij��ѧС����ʵ��������CaSO4��NH3��CO2�Ʊ�(NH4)2SO4������CO2��ˮ���ܽ�Ƚ�С����NH3������ˮ��Ӧ��ͨ������NH3��ʹ��Һ�ʼ��ԣ�Ȼ����ͨ������CO2������XΪNH3��YΪCO2�����ݷ�Ӧ����ͼ���Կ����ں�����Ӧ����������CO2��NH3�����Կ�ѭ�����õ����ʵ���CO��NH3��
��4����õ��Լ����Ȼ�����Һ����Ϊ�ټ����Ȼ�����Һ�����BaSO4��������������������ᱵ�Ķ�Ӧ��ϵΪ��(NH4)2SO4 ~ BaSO4��������淋Ĵ���=16.31g��132/233��10.0g��100%=92.4%
��5���ס�ֱ�Ӽ���NH4Cl���� NH3��HCl�����Թܿڸ���NH3��HCl��Ӧ��������NH4Cl�����Բ���������ȡNH3�������ҡ�����Ũ��ˮ�ٽ�NH3�Ļӷ�������ȡNH3����ȷ�����������������ƺ��Ȼ�粒��壬�������ֽⷴӦ����NH3����������ȡNH3����ȷ������Ũ��ˮ�μӵ�NaOH�����У��ɻӷ���NH3����������ȡNH3����ȷ����ΪҪ�ռ������NH3������a���de����ʯ�Ҹ���NH3����������gf���ռ�NH3����������cb��ͨ��ϡ���ᣬ��ȡ����泥���������h������β����������NH3ֱ��ͨ��ϡ�����лᷢ����������NH3������CCl4������CCl4�������Ƿ�������

��ϰ��ϵ�д�
 ����ʦ���һ��һ��ϵ�д�
����ʦ���һ��һ��ϵ�д�
�����Ŀ

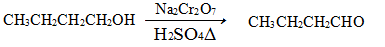

 )��2.50��10��4 mol��L��1����Ca2��_ _____(��ǡ���)������ȫ��[��֪c(Ca2��)��10��5 mol��L��1����Ϊ������ȫ��Ksp(CaCO3)��4.96��10��9]
)��2.50��10��4 mol��L��1����Ca2��_ _____(��ǡ���)������ȫ��[��֪c(Ca2��)��10��5 mol��L��1����Ϊ������ȫ��Ksp(CaCO3)��4.96��10��9]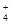 ) c(NO
) c(NO ) ���� > ��< �� = ����(NH4)2CO3��Һ������Ũ���ɴ�С��˳��Ϊ ��
) ���� > ��< �� = ����(NH4)2CO3��Һ������Ũ���ɴ�С��˳��Ϊ ��