��Ŀ����
��ˮFeCl3���غ�ɫ�����׳��⣬100������ʱ��������ҵ�ϳ������л��ϳɴ�����ʵ���ҿ�������װ��(�г�������ȥ)�Ʊ����ռ���ˮFeCl3��
��ش�
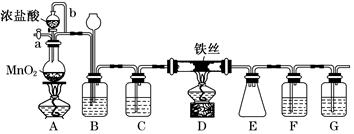
(1)װ��A�з�Ӧ�����ӷ���ʽΪ__________________________________________��
(2)װ��F�����ӵ��Լ�Ϊ_________________________________________________��
(3)����b������Ϊ_____________________________________________________��
װ��B������Ϊ________________________________________________________��
(4)ʵ��ʱӦ�ȵ�ȼA���ľƾ��ƣ���Ӧһ������ٵ�ȼD���ľƾ��ƣ�ԭ��Ϊ_______________________________________________________��
(5)��Ӧ������жװ��ǰ��������еIJ�����_________________________________
(6)Ϊ�������ò�Ʒ���Ƿ���FeCl2���ɽ�������ʵ�飺ȡE���ռ��IJ�����������ˮ�ܽ⣬��������Һ�м���һ���Լ������Լ�Ϊ________(�����)��
��Fe�ۡ���KSCN��Һ��������KMnO4��Һ����NaOH��Һ

��ش�
(1)װ��A�з�Ӧ�����ӷ���ʽΪ__________________________________________��
(2)װ��F�����ӵ��Լ�Ϊ_________________________________________________��
(3)����b������Ϊ_____________________________________________________��
װ��B������Ϊ________________________________________________________��
(4)ʵ��ʱӦ�ȵ�ȼA���ľƾ��ƣ���Ӧһ������ٵ�ȼD���ľƾ��ƣ�ԭ��Ϊ_______________________________________________________��
(5)��Ӧ������жװ��ǰ��������еIJ�����_________________________________
(6)Ϊ�������ò�Ʒ���Ƿ���FeCl2���ɽ�������ʵ�飺ȡE���ռ��IJ�����������ˮ�ܽ⣬��������Һ�м���һ���Լ������Լ�Ϊ________(�����)��
��Fe�ۡ���KSCN��Һ��������KMnO4��Һ����NaOH��Һ
(1)MnO2��4H����2Cl�� Mn2����Cl2����2H2O��(2)Ũ�����ŨH2SO4��(3)ƽ��ѹǿ(��ʹŨ����˳������)����ȥCl2�е�HCl���壬�ж�ʵ������е����Ƿ�Ʒ������(4)�ž�ʵ��װ���еĿ���
Mn2����Cl2����2H2O��(2)Ũ�����ŨH2SO4��(3)ƽ��ѹǿ(��ʹŨ����˳������)����ȥCl2�е�HCl���壬�ж�ʵ������е����Ƿ�Ʒ������(4)�ž�ʵ��װ���еĿ���
(5)����a��ͨ��������ų�װ���ڲ�����Cl2����֤���ո�����(���������𰸾���)��(6)��
 Mn2����Cl2����2H2O��(2)Ũ�����ŨH2SO4��(3)ƽ��ѹǿ(��ʹŨ����˳������)����ȥCl2�е�HCl���壬�ж�ʵ������е����Ƿ�Ʒ������(4)�ž�ʵ��װ���еĿ���
Mn2����Cl2����2H2O��(2)Ũ�����ŨH2SO4��(3)ƽ��ѹǿ(��ʹŨ����˳������)����ȥCl2�е�HCl���壬�ж�ʵ������е����Ƿ�Ʒ������(4)�ž�ʵ��װ���еĿ���(5)����a��ͨ��������ų�װ���ڲ�����Cl2����֤���ո�����(���������𰸾���)��(6)��
(1)MnO2��Ũ���ᷴӦ�����ӷ���ʽΪMnO2��4H����2Cl�� Mn2����Cl2����2H2O��(2)Fװ�����ڷ�ֹ����ˮ�⣬����F��ʢ��Ũ���ᡣ(3)����b��������ƽ��ѹǿ��ʹŨ����˳�����£�װ��B�������dz�ȥCl2�е�HCl���壬ͬʱ�ж�ʵ������е����Ƿ�Ʒ������(4)װ���еĿ�����Ӱ���Ʒ�Ĵ��ȣ����Ե�ȼD���ľƾ���ǰ�����ž�ʵ��װ���еĿ�����(5)�����ж����ڲ�жװ��ǰ���뱻��ȫ���ա�(6)�������Ը��������Һ�����������ӡ�
Mn2����Cl2����2H2O��(2)Fװ�����ڷ�ֹ����ˮ�⣬����F��ʢ��Ũ���ᡣ(3)����b��������ƽ��ѹǿ��ʹŨ����˳�����£�װ��B�������dz�ȥCl2�е�HCl���壬ͬʱ�ж�ʵ������е����Ƿ�Ʒ������(4)װ���еĿ�����Ӱ���Ʒ�Ĵ��ȣ����Ե�ȼD���ľƾ���ǰ�����ž�ʵ��װ���еĿ�����(5)�����ж����ڲ�жװ��ǰ���뱻��ȫ���ա�(6)�������Ը��������Һ�����������ӡ�
 Mn2����Cl2����2H2O��(2)Fװ�����ڷ�ֹ����ˮ�⣬����F��ʢ��Ũ���ᡣ(3)����b��������ƽ��ѹǿ��ʹŨ����˳�����£�װ��B�������dz�ȥCl2�е�HCl���壬ͬʱ�ж�ʵ������е����Ƿ�Ʒ������(4)װ���еĿ�����Ӱ���Ʒ�Ĵ��ȣ����Ե�ȼD���ľƾ���ǰ�����ž�ʵ��װ���еĿ�����(5)�����ж����ڲ�жװ��ǰ���뱻��ȫ���ա�(6)�������Ը��������Һ�����������ӡ�
Mn2����Cl2����2H2O��(2)Fװ�����ڷ�ֹ����ˮ�⣬����F��ʢ��Ũ���ᡣ(3)����b��������ƽ��ѹǿ��ʹŨ����˳�����£�װ��B�������dz�ȥCl2�е�HCl���壬ͬʱ�ж�ʵ������е����Ƿ�Ʒ������(4)װ���еĿ�����Ӱ���Ʒ�Ĵ��ȣ����Ե�ȼD���ľƾ���ǰ�����ž�ʵ��װ���еĿ�����(5)�����ж����ڲ�жװ��ǰ���뱻��ȫ���ա�(6)�������Ը��������Һ�����������ӡ�
��ϰ��ϵ�д�
�����Ŀ
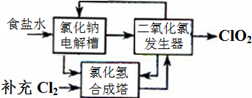
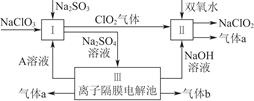
 2ClO2��+2Na2SO4��H2O
2ClO2��+2Na2SO4��H2O H++OH-�� ________________�������ӷ���ʽ��ʾ��.
H++OH-�� ________________�������ӷ���ʽ��ʾ��. C
C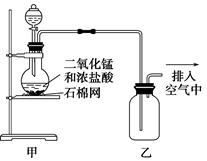
 MnCl2+2H2O+Cl2��
MnCl2+2H2O+Cl2�� CaCl2+2H2O
CaCl2+2H2O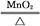 2KCl+3O2��
2KCl+3O2��