��Ŀ����
��������Խ��Խ�ܵ����ǵĹ�ע���±�����ʡ���ֳ��п��������ܱ����������������������������������� ��Ⱦָ���������������������� ��Ҫ��Ⱦ������������������ ���������ȼ�
�������������������������������� 98���������������������������� SO2���������������������������� ��
�ൺ���������������������������� 47���������������������������������������������������������������� ��
�Ͳ����������������������������� 103���������������������������� TSP���������������������������� ��
������������������������������ 90���������������������������� NOx���������������������������� ��
ע��TSP�������������NOx����������
��1�����ϱ���֪�������׳�������ij�����________��
��2�������������׳�������������е�SO2��O2�Ӵ�ʱ��SO2�Ჿ��ת��ΪSO3�����������������������________��������ţ�
A������������������������������������������������������������ B����ԭ��
C���������������������������������������������������������� D��������
��3������������Ҳ�Ǵ�����Ⱦ��֮һ��Ŀǰ������NO�ķ�������һ���������ð���һ��������ԭΪ������ˮ����ѧ�������룬����ͬ�����¿ɲ��ü۸��NH3�����˵���Ȼ��������NO���ɵõ�ͬ��Ч����д���÷�Ӧ�Ļ�ѧ����ʽ_________________________________________________________________________��
��4��ij�еȳ���ÿ��ȼúԼ3´106t���京������1.00%���㣬����ȫ��ת��ΪSO2��SO2��60.0%ת��Ϊ���ᣬ�൱�����ɶ��ٶ�98.0%�����
����ʽ���㣩
������
| ��1������
��2��C ��3��4NO+CH4��2N2+CO2+2H2O ��4����S�غ�ɵù�ϵʽ�� S 32.0 98.0 300´104´1.00%´60.0%t m[H2SO4(aq)]��98.0% m[ =5.63´104t
|

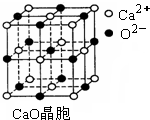
 �� д���������ӵĻ�̬�����Ų�ʽ ��
�� д���������ӵĻ�̬�����Ų�ʽ ��