��Ŀ����
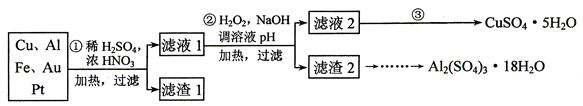
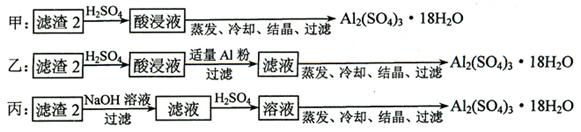
��12�֣���ʳ���г����и����ӡ�þ���ӡ���������ӵȿ����������⣬��������ɳ�Ȳ��������ʡ�����ʳ�õľ������ô�ʳ���ᴿ���õ��ġ�ͨ���������²��裺�ټ����Թ�����BaCl2 ��Һ���ڼ����Թ�����NaOH��Һ���ۼ����Թ�����Na2CO3��Һ���ܹ��ˣ��ݵ���ϡ�����������ݲ�����
�ش��������⡣
��1��ʵ���ҽ���NaCl��Һ����ʱ��һ�������²������̢ٷ��þƾ��ƣ��ڹ̶���Ȧλ�ã��۷�����������������ʢ��NaCl��Һ�����ܼ��Ƚ���;��ֹͣ���ȡ�����ȷ�IJ���˳��Ϊ
��2�����������������鲽��ٺ���Һ������SO42�����ӣ� ������У�Ӧ����γ�ȥSO42�����ӣ� ��
��3������ۼ����Թ���Na2CO3��Һ��ֱ�����ٲ�������Ϊֹ�������ⲽ������Ŀ���� ��
��4������������ܹ��˲�����������һ�����ܳ�����Щ���ʣ�
��
��5��ʵ�����ォ�����Ƴɾ��εĹ����У����ܽ⡢���ˡ�������������IJ����ж�Ҫ�õ���������ʹ�ò�������Ŀ�ģ� ��
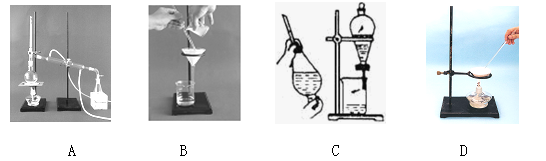
�ش��������⡣
��1��ʵ���ҽ���NaCl��Һ����ʱ��һ�������²������̢ٷ��þƾ��ƣ��ڹ̶���Ȧλ�ã��۷�����������������ʢ��NaCl��Һ�����ܼ��Ƚ���;��ֹͣ���ȡ�����ȷ�IJ���˳��Ϊ
��2�����������������鲽��ٺ���Һ������SO42�����ӣ� ������У�Ӧ����γ�ȥSO42�����ӣ� ��
��3������ۼ����Թ���Na2CO3��Һ��ֱ�����ٲ�������Ϊֹ�������ⲽ������Ŀ���� ��
��4������������ܹ��˲�����������һ�����ܳ�����Щ���ʣ�
��
��5��ʵ�����ォ�����Ƴɾ��εĹ����У����ܽ⡢���ˡ�������������IJ����ж�Ҫ�õ���������ʹ�ò�������Ŀ�ģ� ��
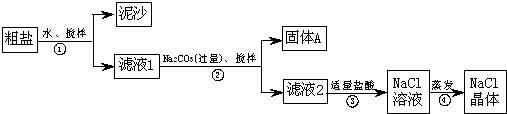
��12�֣���1���٢ڢۢܢ�
��2����ֹƬ�����ϲ���Һ�����μ�һ��BaCl2��Һ��������ְ�ɫ���ǣ�����Һ����SO42������ȥSO42���ķ���������Һ�м��������BaCl2��Һ��
��3����ȥCa2+������Ba2+
��4��BaSO4��CaCO3��Mg(OH)2��BaCO3����ɳ��5�����裬������
��2����ֹƬ�����ϲ���Һ�����μ�һ��BaCl2��Һ��������ְ�ɫ���ǣ�����Һ����SO42������ȥSO42���ķ���������Һ�м��������BaCl2��Һ��
��3����ȥCa2+������Ba2+
��4��BaSO4��CaCO3��Mg(OH)2��BaCO3����ɳ��5�����裬������
�������ʵķ�����ᴿ��
��1������ʱ������ִ�������ʱ����Ӧ��ֹͣ���ȣ�������ȷ��˳���Ǣ٢ڢۢܢݡ�
��2��������Һ������SO42�����ӵķ����Ǽ����μ��Ȼ�����Һ������ֹƬ�����ϲ���Һ�����μ�һ��BaCl2��Һ��������ְ�ɫ���ǣ�����Һ����SO42������ȥSO42���ķ���������Һ�м��������BaCl2��Һ��
��3��������Һ�к��и����Ӽ������ı����ӣ�����̼���Ƶ������dz�ȥCa2+������Ba2+
��
��4�����ڸ�����ת��Ϊ̼��ƣ�þ����ת��Ϊ������þ��SO42��ת��Ϊ���ᱵ����˹��˳�ȥ��������BaSO4��CaCO3��Mg(OH)2��BaCO3����ɳ��
��5�����ܽ������ʱ����Ҫ���������裬������ʱ�������������á�
��1������ʱ������ִ�������ʱ����Ӧ��ֹͣ���ȣ�������ȷ��˳���Ǣ٢ڢۢܢݡ�
��2��������Һ������SO42�����ӵķ����Ǽ����μ��Ȼ�����Һ������ֹƬ�����ϲ���Һ�����μ�һ��BaCl2��Һ��������ְ�ɫ���ǣ�����Һ����SO42������ȥSO42���ķ���������Һ�м��������BaCl2��Һ��
��3��������Һ�к��и����Ӽ������ı����ӣ�����̼���Ƶ������dz�ȥCa2+������Ba2+
��
��4�����ڸ�����ת��Ϊ̼��ƣ�þ����ת��Ϊ������þ��SO42��ת��Ϊ���ᱵ����˹��˳�ȥ��������BaSO4��CaCO3��Mg(OH)2��BaCO3����ɳ��
��5�����ܽ������ʱ����Ҫ���������裬������ʱ�������������á�

��ϰ��ϵ�д�
�����Ŀ