��Ŀ����
ij��ȤС��̽��SO2���廹ԭFe3+��I2������ʹ�õ�ҩƷ��װ������ͼ��ʾ
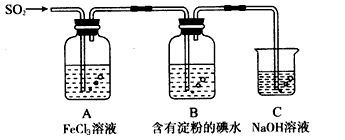
�밴Ҫ��ش���������
(1)����ʵ�鷽����������ʵ������ȡ����SO2����_______��
A. Na2SO3��Һ��HNO3
B. Na2SO3������Ũ����
C. �������ڴ�����ȼ��
D. ��������������
(2)װ��C��������____________��
(3)��Ҫ��A��������Һ��ȡ���壬������е�ʵ��������裺����Ũ������ȴ�ᾧ��________��ϴ�ӡ���Ȼ�������һϵ�в�����û���õ��������У�����ţ�____��
A.������ B.��Һ©�� C.©�� D.������ E.������
(4)Ϊ����֤A��SO2��Fe3+������������ԭ��Ӧ������ͼװ����ͨ�������SO2��ȡA�е���Һ���ֳ����ݣ������������ʵ�飺
�����٣�����һ����Һ�м�������KMnO4��Һ����ɫ��ȥ��
�����ڣ����ڶ�����Һ�м���KSCN��Һ����죬�ټ������Ƶ���ˮ����Һ��죻
�����ۣ�����������Һ�м����������ữ��BaCl2��Һ��������ɫ������
�����������ķ�����____________��ԭ����__________________��
(5)��ʵ�����ܱ���SO2�Ļ�ԭ�Ա�I-�Ļ�ԭ��ǿ��������__________________���䷴Ӧ�����ӷ���ʽΪ
______________��
(1)����ʵ�鷽����������ʵ������ȡ����SO2����_______��
A. Na2SO3��Һ��HNO3
B. Na2SO3������Ũ����
C. �������ڴ�����ȼ��
D. ��������������
(2)װ��C��������____________��
(3)��Ҫ��A��������Һ��ȡ���壬������е�ʵ��������裺����Ũ������ȴ�ᾧ��________��ϴ�ӡ���Ȼ�������һϵ�в�����û���õ��������У�����ţ�____��
A.������ B.��Һ©�� C.©�� D.������ E.������
(4)Ϊ����֤A��SO2��Fe3+������������ԭ��Ӧ������ͼװ����ͨ�������SO2��ȡA�е���Һ���ֳ����ݣ������������ʵ�飺
�����٣�����һ����Һ�м�������KMnO4��Һ����ɫ��ȥ��
�����ڣ����ڶ�����Һ�м���KSCN��Һ����죬�ټ������Ƶ���ˮ����Һ��죻
�����ۣ�����������Һ�м����������ữ��BaCl2��Һ��������ɫ������
�����������ķ�����____________��ԭ����__________________��
(5)��ʵ�����ܱ���SO2�Ļ�ԭ�Ա�I-�Ļ�ԭ��ǿ��������__________________���䷴Ӧ�����ӷ���ʽΪ
______________��
(1)B
(2)��ȥβ���е�SO2
(3)���ˣ�BE
(4)�����٣���ΪSO2������ˮ��A��Һ�к��е�SO2Ҳ��ʹKMnO4��Һ��ɫ
(5)B����Һ����ɫ��ȥ��SO2+I2+2H2O=SO42-+2I-+4H+
(2)��ȥβ���е�SO2
(3)���ˣ�BE
(4)�����٣���ΪSO2������ˮ��A��Һ�к��е�SO2Ҳ��ʹKMnO4��Һ��ɫ
(5)B����Һ����ɫ��ȥ��SO2+I2+2H2O=SO42-+2I-+4H+

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

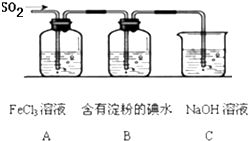 ij��ȤС��̽��SO2���廹ԭFe3+��I2������ʹ�õ�ҩƷ��װ����ͼ��ʾ��
ij��ȤС��̽��SO2���廹ԭFe3+��I2������ʹ�õ�ҩƷ��װ����ͼ��ʾ��

