��Ŀ����
CO��H2�Ļ�������׳ƺϳ�������һ����Ҫ�Ĺ�ҵԭ��������̿����Ȼ������Ҫ�ɷ�ΪCH4�������͡�ú�ڸ����¾�����ˮ������Ӧ�Ƶúϳ�����
��1����֪ij��Ӧ��ƽ�ⳣ������ʽΪ��K=
��������Ӧ�Ļ�ѧ����ʽΪ�� ��
��2�������Ϊ2L���ܱ������г���CH4��H2O��g����ɵĻ��������1mol������һ�������·�����Ӧ�����ﵽƽ��״̬������¶ȡ�ѹǿ��Ͷ�ϱ�X��
�Ը÷�Ӧ��Ӱ����ͼ��ʾ��
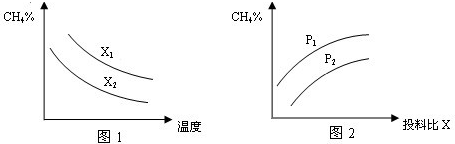
��ͼ1�е�����������ʾͶ�ϱȵĹ�ϵX2 X1���=��������������ͬ��
��ͼ2������������ʾ��ѹǿ�ȵĹ�ϵ��P2 P1
��3����CH4��O2Ϊԭ�Ͽ���Ƴ�ȼ�ϵ�أ�
����װ����ʢ��150.0mL 1.0mol/L KOH��Һ���ŵ�ʱ���뷴Ӧ�������ڱ�״���µ����Ϊ4.48L������ŵ������û�������ݳ�����ŵ���Ϻ�������Һ�и�����Ũ���ɴ�С�Ĺ�ϵΪ�� ��
����H2SO4��Һ����KOH��ҺΪ�������Һ����CH4��ΪC6H12O6����ȼ�ϵ�صĸ�����ӦʽΪ�� ��
��1����֪ij��Ӧ��ƽ�ⳣ������ʽΪ��K=
| c(H2)?c(CO) |
| c(H2O) |
��2�������Ϊ2L���ܱ������г���CH4��H2O��g����ɵĻ��������1mol������һ�������·�����Ӧ�����ﵽƽ��״̬������¶ȡ�ѹǿ��Ͷ�ϱ�X��
| n(CH4) |
| n((H2O) |

��ͼ1�е�����������ʾͶ�ϱȵĹ�ϵX2
��ͼ2������������ʾ��ѹǿ�ȵĹ�ϵ��P2
��3����CH4��O2Ϊԭ�Ͽ���Ƴ�ȼ�ϵ�أ�
����װ����ʢ��150.0mL 1.0mol/L KOH��Һ���ŵ�ʱ���뷴Ӧ�������ڱ�״���µ����Ϊ4.48L������ŵ������û�������ݳ�����ŵ���Ϻ�������Һ�и�����Ũ���ɴ�С�Ĺ�ϵΪ��
����H2SO4��Һ����KOH��ҺΪ�������Һ����CH4��ΪC6H12O6����ȼ�ϵ�صĸ�����ӦʽΪ��
���㣺����ٷֺ������¶ȡ�ѹǿ�仯����,��ѧ��Դ���͵��
ר�⣺��ѧƽ��ר��,�绯ѧר��
��������1����ѧƽ�ⳣ��ָ��һ���¶��£����淴Ӧ�ﵽƽ��ʱ����������Ũ�ȵĻ�ѧ���������ݵij˻����Ը���Ӧ��Ũ�ȵĻ�ѧ���������ݵij˻����õı�ֵ������Ԫ���غ㣬����һ��Ӧ��Ϊ����C���ݴ���д����ʽ��
��2����Ͷ�ϱ�X=
Խ��ƽ��ʱ�����ת����Խ�ͣ�����Խ�ߣ�
��Ͷ�ϱ�һ��ʱ������ѹǿ��ƽ���ƶ�Ӱ�죬���ͼ��������
��3���ټ������������ʵ����������������ɶ�����̼�����ʵ���������n��KOH����n��CO2��������ϵ�жϷ�Ӧ�������������Һ�е�������ʵ������������ˮ���������жϣ�
��ʧ���ӵ������ڸ���ͨ�룬����������Ӧ�������ϵõ����ӷ�����ԭ��Ӧ���������Һ������Һ����Ӧ��C6H12O6ʧ�������ɶ�����̼�������ӣ�
��2����Ͷ�ϱ�X=
| n(CH4) |
| n((H2O) |
��Ͷ�ϱ�һ��ʱ������ѹǿ��ƽ���ƶ�Ӱ�죬���ͼ��������
��3���ټ������������ʵ����������������ɶ�����̼�����ʵ���������n��KOH����n��CO2��������ϵ�жϷ�Ӧ�������������Һ�е�������ʵ������������ˮ���������жϣ�
��ʧ���ӵ������ڸ���ͨ�룬����������Ӧ�������ϵõ����ӷ�����ԭ��Ӧ���������Һ������Һ����Ӧ��C6H12O6ʧ�������ɶ�����̼�������ӣ�
���
�⣨1��ƽ�����ʽΪ��K=
��������ΪCO��H2����Ӧ�ﺬ��H2O������ѧ�������ֱ�Ϊ1��1��1������Ԫ���غ㣬����һ��Ӧ��Ϊ����C����Ӧ��������Ӧ��Ӧ�Ļ�ѧ����ʽΪ��C��s��+H2O��g��?CO��g��+H2��g����
�ʴ�Ϊ��C��s��+H2O��g��?CO��g��+H2��g����
��2����Ӧ����ʽΪ��CH4��g��+H2O��g��=CO��g��+3H2��g����
���ϱ�X=
Խ��ƽ��ʱ�����ת����Խ�ͣ�����Խ�ߣ���x2��x1���ʴ�Ϊ������
�ڸ÷�Ӧ����Ӧ�������������ķ�Ӧ������ѹǿƽ�����淴Ӧ�����ƶ���ƽ��ʱ����ĺ�������p2��p1���ʴ�Ϊ������
��3���ٲ��뷴Ӧ�������ڱ�״�������Ϊ4.48L�����ʵ���Ϊ
=0.2mol�����ݵ���ת���غ��֪�����ɶ�����̼Ϊ
=0.1mol��n��KOH��=0.15L��1.0mol?L-1=0.15mol��n��KOH����n��CO2��=0.15mol��0.1mol=3��2����������2CO2+3KOH=K2CO3+KHCO3+H2O����Һ��̼���ˮ�⣬̼�������ˮ����ڵ��룬��Һ�ʼ��ԣ���c��OH-����c��H+����̼�����ˮ��̶ȴ���̼���������c��HCO3-����c��CO32-����������Ũ�����ˮ��̶Ȳ���̼���Ũ��ԭ�������������ӣ���c��K+����c��HCO3-����c��CO32-����c��OH-����c��H+����
�ʴ�Ϊ��c��K+����c��HCO3-����c��CO32-����c��OH-����c��H+����
��ԭ�����ʧ���ӵ�����������������Ӧ�������ϵõ����ӷ�����ԭ��Ӧ����������Һ�У�C6H12O6ʧ�������ɶ�����̼�������ӣ������缫��ӦʽΪC6H12O6-24e-+6H2O=6CO2+24H+��
�ʴ�Ϊ��C6H12O6-24e-+6H2O=6CO2+24H+��
| c(H2)?c(CO) |
| c(H2O) |
�ʴ�Ϊ��C��s��+H2O��g��?CO��g��+H2��g����
��2����Ӧ����ʽΪ��CH4��g��+H2O��g��=CO��g��+3H2��g����
���ϱ�X=
| n(CH4) |
| n((H2O) |
�ڸ÷�Ӧ����Ӧ�������������ķ�Ӧ������ѹǿƽ�����淴Ӧ�����ƶ���ƽ��ʱ����ĺ�������p2��p1���ʴ�Ϊ������
��3���ٲ��뷴Ӧ�������ڱ�״�������Ϊ4.48L�����ʵ���Ϊ
| 4.48L |
| 22.4L/mol |
| 0.2mol��4 |
| 8 |
�ʴ�Ϊ��c��K+����c��HCO3-����c��CO32-����c��OH-����c��H+����
��ԭ�����ʧ���ӵ�����������������Ӧ�������ϵõ����ӷ�����ԭ��Ӧ����������Һ�У�C6H12O6ʧ�������ɶ�����̼�������ӣ������缫��ӦʽΪC6H12O6-24e-+6H2O=6CO2+24H+��
�ʴ�Ϊ��C6H12O6-24e-+6H2O=6CO2+24H+��
�����������ۺ��Խϴ��漰ƽ�ⳣ������ѧƽ��ͼ��Ӱ�����ء�ԭ��ء���ѧ���㡢����Ũ�ȱȽϵȣ�Ϊ�߿��������ͣ��ǶԻ���֪ʶ��ѧ���������ۺϿ��飬ע����ջ�ѧƽ���Ӱ�������Լ�ͼ�����ݵķ�����������������Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
���������ۻ�ʱ�����ƻ���ѧ�����ǣ�������
| A���Ȼ��� | B���ɱ� |
| C�����ʯ | D��ˮ�� |
 ���ܱ������У���ӦX2��g��+Y2��g���TXY��g����H��0���ﵽ��ƽ�⣮�ڽ��ı�ijһ�����ﵽ��ƽ�⣬�Դ˹��̵ķ�����ȷ���ǣ�������
���ܱ������У���ӦX2��g��+Y2��g���TXY��g����H��0���ﵽ��ƽ�⣮�ڽ��ı�ijһ�����ﵽ��ƽ�⣬�Դ˹��̵ķ�����ȷ���ǣ�������| A��ͼI�������¶ȵı仯��� |
| B��ͼ����������������ı仯��� |
| C��ͼ��������ѹǿ�ı仯��� |
| D��ͼ���������¶ȵı仯��� |
���з�Ӧ���ӷ���ʽ��ȷ���ǣ�������
| A������ʯ��ˮ��̼����Ʒ�Ӧ��Ca2++2HCO3-+2OH-�TCaCO3��+CO32-+2H2O |
| B��FeSO4������Һ��¶�ڿ����У�4Fe2++O2+4H+�T4Fe3++2H2O |
| C�����Ȼ�����Һ�еμӹ�����ˮ��Al3++4NH3?H2O�TAlO42-+4NH4++2H2O |
| D��H2SO4��Ba��OH��2��Һ��Ӧ��Ba2++OH-+H++SO42-�TBaSO4��+H2O |
���и������ʲ���ʵ��ֱ��ת�����ǣ�������
| A��S��SO2��H2SO4��MgSO4 |
| B��Cu��CuCl2��Cu��NO3��2��Cu��OH��2 |
| C��Al��Al2O3��Al��OH��3��NaAlO2 |
| D��Na��Na2O��Na2CO3��NaCl |


 ��1�����ӱ��еĵ�Դ��������пԭ��أ���缫�ֱ�ΪAg2O��Zn���������ҺΪKOH��Һ���ܷ�ӦʽΪ��Ag2O+H2O+Zn�TZn��OH��2+2Ag����ش𣺷ŵ�ʱ�������缫�ϵķ�ӦʽΪ��
��1�����ӱ��еĵ�Դ��������пԭ��أ���缫�ֱ�ΪAg2O��Zn���������ҺΪKOH��Һ���ܷ�ӦʽΪ��Ag2O+H2O+Zn�TZn��OH��2+2Ag����ش𣺷ŵ�ʱ�������缫�ϵķ�ӦʽΪ��