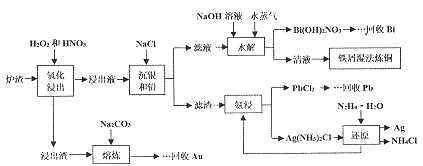
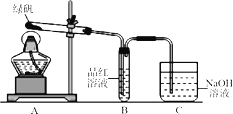
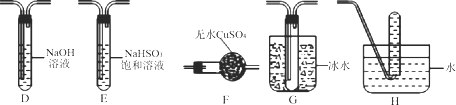
��Ŀ����
����Ŀ��A��������Ҫ��Ӫ�����ʣ�����Ȼ�л��߷��ӻ����D��һ����Ҫ�Ļ���ԭ�ϡ�����ͬ�����£�G�������ܶ���E�����ܶȵ�2��������֮���ת����ϵ��ͼ��

(1)���Թ��м���2mL10%����������Һ���μ�4-5��5%����ͭ��Һ�������2mL10%��E����Һ�����ȣ��ɹ۲쵽��������___________��
(2)д��C��F��ŨH2SO4����������G�Ļ�ѧ����ʽ________���÷�Ӧ��������______��
(3)��֪D��F�ڴ��������·�ӦҲ������G��д���÷�Ӧ�Ļ�ѧ����ʽ______��
(4)����˵������ȷ����________��
a.Ҫȷ��A�ѷ�������ˮ�⣬�Ƚ�ˮ��Һ�кͳɼ��ԣ�����������Һ�͵�ˮ�ֱ����
b.�л���B��C��D��G����ʹ����KMnO4��Һ��ɫ
c.��������Dͨ����ˮ�У����Թ۲쵽��ˮ��ɫ����Һ�ֲ�
d.ij��X����Է���������D��H֮�ͣ���X������ˮ�����ӳɷ�Ӧ
(5)д����G��һ��̼ԭ�ӵ�ͬϵ��Ŀ��ܽṹ��ʽ_________��
���𰸡� ��Һ������ש��ɫ���� CH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O ȡ����Ӧ CH2=CH2+CH3COOH
CH3COOCH2CH3+H2O ȡ����Ӧ CH2=CH2+CH3COOH![]() CH3COOCH2CH3 abd HCOOCH2CH3��CH3COOCH3
CH3COOCH2CH3 abd HCOOCH2CH3��CH3COOCH3
��������A��������Ҫ��Ӫ�����ʣ�����Ȼ�л��߷��ӻ�������AΪ���ۣ�������������ˮ������������B��B�ھ���ø�����·�Ӧ����CΪ�Ҵ���HΪ������̼��D��һ����Ҫ�Ļ���ԭ�ϣ�����ˮ��Ӧ�����Ҵ���DΪ��ϩ����ϩ�����õ�E������ͬ�����£�G�������ܶ���E�����ܶȵ�2�����ݴ˷����ɵ�EΪ��ȩ��FΪ���ᣬ�Ҵ������ᷢ��������Ӧ����GΪ����������
(1)���Թ��м���2mL10%����������Һ���μ�4-5��5%����ͭ��Һ�������2mL10%��E����Һ����ȩ��Һ�����ȣ��ɹ۲쵽����������Һ������ש��ɫ������(2) C��F��ŨH2SO4����������G�Ļ�ѧ����ʽΪCH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O���÷�Ӧ��������������Ӧ��ȡ����Ӧ��(3) D��F�ڴ��������·�ӦҲ������G����Ӧ�Ļ�ѧ����ʽΪCH2=CH2+CH3COOH
CH3COOCH2CH3+H2O���÷�Ӧ��������������Ӧ��ȡ����Ӧ��(3) D��F�ڴ��������·�ӦҲ������G����Ӧ�Ļ�ѧ����ʽΪCH2=CH2+CH3COOH![]() CH3COOCH2CH3��(4) a.Ҫȷ��A�����ۣ��ѷ�������ˮ�⣬�ȼӵ�ˮ�����Ƿ��е��ۣ��ٽ�ˮ��Һ�кͳɼ��ԣ�����������Һ�����Ƿ���ˮ����������ǣ��ʲ���ȷ��b.�л���B��C��D�ֱ����ǻ���ȩ����̼̼˫��������ʹ����KMnO4��Һ��ɫ����GΪ������������ʹ���Ը��������Һ��ɫ���ʲ���ȷ��c.��������Dͨ����ˮ�У����Թ۲쵽��ˮ��ɫ�����ɵ�1��2-����������ˮ�����ܣ���Һ�ֲ㣬����ȷ��d.ij��X����Է���������D��H֮�ͼ�28+44=72��
CH3COOCH2CH3��(4) a.Ҫȷ��A�����ۣ��ѷ�������ˮ�⣬�ȼӵ�ˮ�����Ƿ��е��ۣ��ٽ�ˮ��Һ�кͳɼ��ԣ�����������Һ�����Ƿ���ˮ����������ǣ��ʲ���ȷ��b.�л���B��C��D�ֱ����ǻ���ȩ����̼̼˫��������ʹ����KMnO4��Һ��ɫ����GΪ������������ʹ���Ը��������Һ��ɫ���ʲ���ȷ��c.��������Dͨ����ˮ�У����Թ۲쵽��ˮ��ɫ�����ɵ�1��2-����������ˮ�����ܣ���Һ�ֲ㣬����ȷ��d.ij��X����Է���������D��H֮�ͼ�28+44=72��![]() ����XΪC5H12��Ϊ��������������ˮ�����ӳɷ�Ӧ���ʲ���ȷ����ѡabd��(5) GΪ������������G��һ��̼ԭ�ӵ�ͬϵ��Ŀ��ܽṹ��ʽ��HCOOCH2CH3��CH3COOCH3��
����XΪC5H12��Ϊ��������������ˮ�����ӳɷ�Ӧ���ʲ���ȷ����ѡabd��(5) GΪ������������G��һ��̼ԭ�ӵ�ͬϵ��Ŀ��ܽṹ��ʽ��HCOOCH2CH3��CH3COOCH3��