��Ŀ����
����Ŀ��G��ҩ��ϳ��е�һ����Ҫ�м��壬������G��һ�ֺϳ�·�ߣ�


�ش��������⣺
��1��B�Ľṹ��ʽΪ__________���������������ŵ�����Ϊ__________��B����C�ķ�Ӧ����Ϊ__________��
��2��D��������__________��
��3����C��E�ϳ�F�Ļ�ѧ����ʽΪ________________________________________��
��4��D��ͬ���칹���У��ܷ���������Ӧ�ҷ��ӽṹ�к������Ļ���__________�֣����к˴Ź�����������6��壬�����֮��Ϊ1��1��1��1��1��1��ͬ���칹��Ľṹ��ʽΪ______________________ (һ�ּ���)��
��5�����������ϳ�·�ߣ���CH3CH2ClΪԭ��(�����Լ���ѡ)������Ʊ��Ͷ�ȩ(CH3CH=CH
CHO)�ĺϳ�·�ߡ�_______________
���𰸡� �ǻ����ʻ� ȡ����Ӧ ���ǻ�����ȩ
�ǻ����ʻ� ȡ����Ӧ ���ǻ�����ȩ 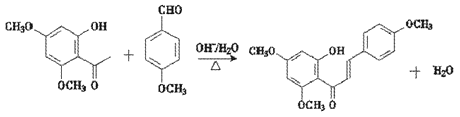 3
3  ��
��
![]()
��������
����A��C�ṹ������ʽ�����𣬽����֪�������ɵõ�B���ʵĽṹ��ʽ���京�еĹ����ŵ����ࣻ������B��C�ṹ�����������B����C�ķ�Ӧ���ͣ������ø÷�Ӧ�������Ʒ�����D�Ľṹ��ʽ������ϵͳ��������D���������ж�D��ͬ���칹��ʱ����ʱ��Ҫ�ӹ������칹��λ���칹���棬������Ҫ���������д����Ӧ�����ʵĽṹ��ʽ������C+E����Fʱ�ı仯������Ԫ�ص�ԭ���غ㣬�ɵø÷�Ӧ�Ļ�ѧ����ʽ���������֪����CH3CH2Cl�������Ŀ��֪��Ϣ���ϳ���Ҫ�Ʊ���Ŀ�����Ͷ�ȩ(CH3CH= CHCHO)��
(1)����A��C�ṹ����������֪��Ϣ��B�ķ���ʽ����֪B���ʵĽṹ��ʽ�� �������ʺ��еĹ��������ǻ����ʻ���B��CH3I����ȡ����Ӧ������C
�������ʺ��еĹ��������ǻ����ʻ���B��CH3I����ȡ����Ӧ������C ��HI��
��HI��
(2)D��CH3I����ȡ����Ӧ�ɲ��� �����ƿ�֪DΪ���ǻ�����ȩ
�����ƿ�֪DΪ���ǻ�����ȩ![]() ��(3)��C
��(3)��C ��E
��E �ڼ��������¼��ȣ���Ӧ����F
�ڼ��������¼��ȣ���Ӧ����F ��ˮ����Ӧ�Ļ�ѧ����ʽΪ
��ˮ����Ӧ�Ļ�ѧ����ʽΪ ��
��
(4)D��![]() ���ж���ͬ���칹�壬���е�ͬ���칹����ӽṹ�к����������ܷ���������Ӧ˵������ȩ��������
���ж���ͬ���칹�壬���е�ͬ���칹����ӽṹ�к����������ܷ���������Ӧ˵������ȩ�������� ��
�� ��
��![]() ����3�֣����к˴Ź�����������6��壬�����֮��Ϊ1��1��1��1��1��1��ͬ���칹��Ľṹ��ʽΪ
����3�֣����к˴Ź�����������6��壬�����֮��Ϊ1��1��1��1��1��1��ͬ���칹��Ľṹ��ʽΪ ��
�� ��
��
(5)��CH3CH2ClΪԭ���Ʊ��Ͷ�ȩ(CH3CH= CHCHO)��������ˮ��õ��Ҵ����ٰ��Ҵ��������õ���ȩ�������������Ϣ�ϳɰͶ�ȩ������ϳ�·��Ϊ��![]() ��
��