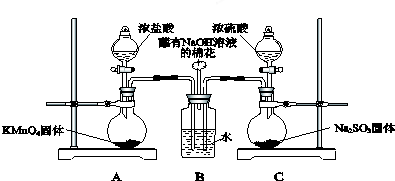
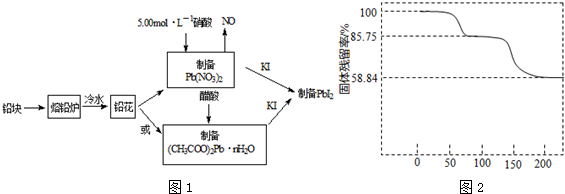
��Ŀ����
3���ں����ܱ������н��кϳɰ���Ӧ��N2��g��+3H2��g���T2NH3��g����H��0����ƽ�ⳣ��K���¶�T�Ĺ�ϵ���£�| T/K | 298 | 398 | 498 |
| ƽ�ⳣ��K/L2•mol-2 | 4.1��106 | K1 | K2 |
��2�����и����˵����Ӧ�ﵽƽ��״̬����ce
a��������N2��H2��NH3Ũ�ȱ�Ϊ1��3��2 b��v��N2����=3v��H2����
c��������ѹǿ���ֲ��� d����������ܶȱ��ֲ���
e���������ƽ��Ħ���������ֲ���
��3��ij�¶��£���1L���º��������г���1mol N2��3mol H2����������Ӧ��10min��ƽ�⣬��ʱ������ѹǿ��Ϊԭ����$\frac{7}{8}$��
�ٸù��̵�ƽ����Ӧ����v��NH3��=0.05mol•L-1•min-1
��N2��ƽ��ת����Ϊ25%��
���� ��1������ӦΪ���ȷ�Ӧ�������¶�ƽ�������ƶ���ƽ�ⳣ����С��
��2�����淴Ӧ����ƽ��ʱ��ͬ�����ʵ�����������ȣ�����ֵ�Ũ�ȡ��������ֲ��䣬�ɴ�����������һЩ�����䣬�ж�ƽ���������Ӧ�淴Ӧ���з����仯�����������ɱ仯�����仯˵������ƽ�⣻
��3����1L���º��������г���1mol N2��3mol H2��10min��ƽ�⣬
N2��g��+3H2��g���T2NH3��g��
��ʼ����mol����1 3 0
ת������mol����x 3x 2x
ƽ������mol����1-x 3-3x 2x
ѹǿ֮�ȵ������ʵ���֮�ȣ��з��̼���x��ֵ������v=$\frac{��c}{��t}$����v��NH3��������ת����=$\frac{���ĵ��������ʵ���}{������ʼ���ʵ���}$��100%��
��� �⣺��1������ӦΪ���ȷ�Ӧ�������¶�ƽ�������ƶ���ƽ�ⳣ����С����ƽ�ⳣ��K1��K2��
�ʴ�Ϊ������
��2��a��������N2��H2��NH3Ũ�ȱ�����ʼŨ�ȼ�ת�����йأ�ƽ��ʱ��һ�����ڻ�ѧ������֮�ȣ���a����
b��ƽ��ʱ��ͬ���ʱ�ʾ����������֮�ȵ��ڻ�ѧ������֮�ȣ�Ӧ��3v��N2����=v��H2����ʱ��Ӧ����ƽ��״̬����b����
c���淴Ӧ���У�����������ʵ����仯��������ѹǿ�����仯��ѹǿ���ֲ���˵������ƽ�⣬��c��ȷ��
d������������������䣬�����ݻ����䣬��������ܶ�ʼ�ձ��ֲ��䣬��d����
e������������������䣬�淴Ӧ���У�����������ʵ����仯����ƽ��Ħ�������仯�����������ƽ��Ħ���������ֲ��䣬˵������ƽ�⣬��e��ȷ��
��ѡ��ce��
��3����1L���º��������г���1mol N2��3mol H2��10min��ƽ�⣬
N2��g��+3H2��g���T2NH3��g��
��ʼ����mol����1 3 0
ת������mol����x 3x 2x
ƽ������mol����1-x 3-3x 2x
ѹǿ֮�ȵ������ʵ���֮�ȣ���$\frac{4-2x}{4}$=$\frac{7}{8}$�����x=0.25��
��v��NH3��=$\frac{\frac{0.5mol}{1L}}{10min}$=0.05mol•L-1•min-1���ʴ�Ϊ��0.05mol•L-1•min-1��
�ڵ���ת����=$\frac{0.25mol}{1mol}$��100%=25%���ʴ�Ϊ��25%��
���� ���⿼�黯ѧƽ�������Ӱ�����ء�ƽ��״̬�жϡ�ƽ�ⳣ��Ӱ�����ء���Ӧ���ʼ��㣬�ѶȲ���ע������ʽ���ⷨ�ڻ�ѧƽ�������Ӧ�ã�

 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�| A�� | K+��CO32-��Cl-��NO3- | B�� | MnO4-��K+��OH-��SO42- | ||
| C�� | Cl-��SO42-��Cu2+��Ba2+ | D�� | Mg2+��Na+��NO3-��Cl- |
| A�� | ������HCl | B�� | ������NaCl | C�� | �����İ�ˮ | D�� | ������NaOH |
| A�� | ���;ƾ� | B�� | ���Ȼ�̼���� | C�� | ����ˮ | D�� | �����ˮ |
| A�� | ���뷽��ʽ��Na2SO4�TNa2++SO42- | B�� | �Ȼ��Ƶĵ���ʽ��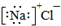 | ||
| C�� | 14C��ԭ�ӽṹʾ��ͼ��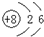 | D�� | CH4���ӵı���ģ�ͣ� |