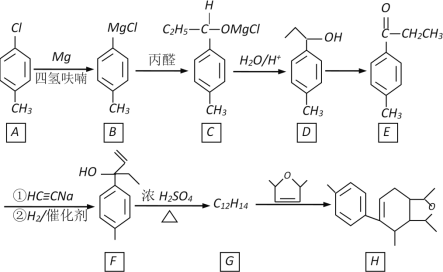
��Ŀ����
����Ŀ����֪Ԫ��A��һ�������ӵĵ��Ӳ�ṹ���ԭ����ͬ��Ԫ��B�Ķ��������ӵĵ��Ӳ�ṹ����ԭ����ͬ����ش�
��1��Ԫ��A���ӵĽṹʾ��ͼΪ___��A�ĵ��ʳ�___ɫ����������ʵķ�����___��
��2����A��B�γɵĻ�����ĵ���ʽ��___��
��3����A���Ӿ�����ͬ��������һ�ַ�����___�������ƣ���
��4��A�ĵ�����Ca(OH)2��Ӧ�Ļ�ѧ����ʽ___���÷�Ӧ��ҵ�ϵ���;��___��
��5����ɫʯ����ֽ����A��ˮ��Һ������___����������������___��
���𰸡�![]() ����ɫ ��ʪ��ĵ��۵⻯����ֽ���飬��ֽ����
����ɫ ��ʪ��ĵ��۵⻯����ֽ���飬��ֽ���� 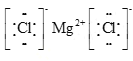 �Ȼ���� 2Cl2+2Ca(OH)2=CaCl2+Ca(ClO)2+2H2O ��ȡƯ�� �ȱ�����ɫ ��ˮ�д���
�Ȼ���� 2Cl2+2Ca(OH)2=CaCl2+Ca(ClO)2+2H2O ��ȡƯ�� �ȱ�����ɫ ��ˮ�д���![]() �����ԣ����ʹ��ֽ��죬��ͬʱ����HClO���ӣ�HClO����ǿ�����ԣ�ʹ��ɫ������ɫ�������ɫ�漴��ʧ
�����ԣ����ʹ��ֽ��죬��ͬʱ����HClO���ӣ�HClO����ǿ�����ԣ�ʹ��ɫ������ɫ�������ɫ�漴��ʧ
��������
��֪Ԫ��A��һ�������ӵĵ��Ӳ�ṹ�����ͬ��AΪCl��Ԫ��B�Ķ��������ӵĵ��Ӳ�ṹ������ͬ��BΪMg��
��1��Ԫ��AΪCl���õ�һ�����ӱ�Ϊ�����ӣ��ṹʾ��ͼΪ ��A�ĵ���Ϊ�������ʻ���ɫ����������ʵķ�������ʪ��ĵ��۵⻯����ֽ���飬��ֽ����˵������������
��A�ĵ���Ϊ�������ʻ���ɫ����������ʵķ�������ʪ��ĵ��۵⻯����ֽ���飬��ֽ����˵������������
��Ϊ��![]() ������ɫ����ʪ��ĵ��۵⻯����ֽ���飬��ֽ������
������ɫ����ʪ��ĵ��۵⻯����ֽ���飬��ֽ������
��2����A��B�γɵĻ�����Ϊ�Ȼ�þ����þ���Ӻ������ӹ��ɣ��������ӻ��������ʽ��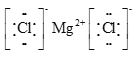 ��
��
����ʽΪ��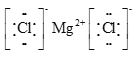 ��
��
��3�������Ӻ�����18�����ӣ��������Ӿ�����ͬ��������һ�ַ����Ȼ��⡢���⡢˫��ˮ�ȡ�
��Ϊ���Ȼ��⣨���⡢˫��ˮ�ȣ�
��4��������Ca(OH)2��Ӧ�����Ȼ��ơ�������ƺ�ˮ����ѧ����ʽ2Cl2+2Ca(OH)2=CaCl2+Ca(ClO)2+2H2O����ҵ�Ͽ��ø÷�Ӧ��ȡƯ�ۣ�
��Ϊ��2Cl2+2Ca(OH)2=CaCl2+Ca(ClO)2+2H2O ����ȡƯ�ۣ�
��5����ˮ�д���![]() �����ԣ����ʹ��ֽ��죬��ͬʱ����HClO���ӣ�HClO����ǿ�����ԣ�ʹ��ɫ������ɫ�������ɫ�漴��ʧ���������Ϊ�ȱ�����ɫ��
�����ԣ����ʹ��ֽ��죬��ͬʱ����HClO���ӣ�HClO����ǿ�����ԣ�ʹ��ɫ������ɫ�������ɫ�漴��ʧ���������Ϊ�ȱ�����ɫ��
��Ϊ���ȱ�����ɫ����ˮ�д���![]() �����ԣ����ʹ��ֽ��죬��ͬʱ����HClO���ӣ�HClO����ǿ�����ԣ�ʹ��ɫ������ɫ�������ɫ�漴��ʧ��
�����ԣ����ʹ��ֽ��죬��ͬʱ����HClO���ӣ�HClO����ǿ�����ԣ�ʹ��ɫ������ɫ�������ɫ�漴��ʧ��

 ��һ������ĩ�ٷֳ�̾�ϵ�д�
��һ������ĩ�ٷֳ�̾�ϵ�д�