��Ŀ����
��ij��ѧ��ȤС����ʵ��ʧ����ȡƯ�ۣ���̽��������ʯ���鷴Ӧ�������Ͳ����֪���ٶ���������Ũ���ᷴӦ���Ʊ�������MnO2 +4HCl(Ũ)=MnCl2+Cl2��+H2O
�������ͼӦΪ���ȷ�Ӧ���¶Ƚϸ�ʱ�������ͼ�ܷ������·�Ӧ�� 6Cl2+6Ca(OH)2 5CaCl2+Ca(ClO3)2+6H2O
5CaCl2+Ca(ClO3)2+6H2O
����ȤС�����������ʵ��װ�ã�����ʵ�顣
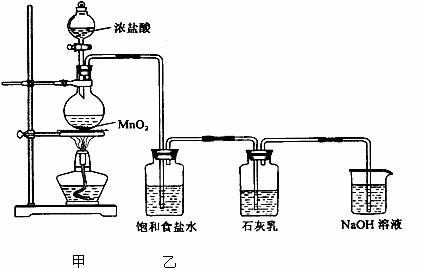
��ش��������⣺
��1���ټ�װ�������Ʊ���������װ�õ�������___________ ��
 �ڸ���ȤС����8.7g MnO2��������Ũ�����ַ�Ӧ�������ռ�����������״���� ���������������������ʯ���鷴Ӧ���������������Ƶ�Ca(ClO)2______________g��
�ڸ���ȤС����8.7g MnO2��������Ũ�����ַ�Ӧ�������ռ�����������״���� ���������������������ʯ���鷴Ӧ���������������Ƶ�Ca(ClO)2______________g��
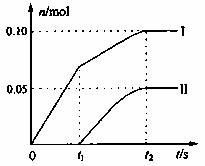
��2��С���Ա���֣�������Ca(ClO)2����������С������ֵ���������ۺ���Ϊ�����¶����ߵ��·�Ӧ�����仯�ǿ���ԭ��Ϊ��̽����Ӧ�����Բ����Ӱ�죬������ȡһ������ʯ���飬�������ٵ�ͨ�������������ó���ClO-��ClO3-�������ӵ����ʵ�����n���뷴Ӧʱ��(t)�Ĺ�ϵ���ߣ����Ա�ʾΪ��ͼ��������������ˮ�ķ�Ӧ����
��ͼ������II��ʾ_____________���ӵ����ʵ����淴Ӧʱ��仯�Ĺ�ϵ��
����ȡʯ�����к���Ca(OH)2�����ʵ���Ϊ______________mol��
��д��������̣�
��3��Ϊ�����Ca(ClO)2�IJ��ʣ��ɶԱ�װ�����ʵ��Ľ����������һ�ָĽ�������________ _��
ʵ������Ҫ����ijЩ����ʱ��ͨ��ʹ�ÿ��ٵķ����Ʊ������м���ʵ��ɿ�����ȡʵ����������������壬�������������ʵ�顣��ʵ��װ������ͼ��ʾ��
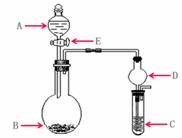 |
��1����A��Ϊ30%��H2O2��Һ��B�й���ΪMnO2 ��C��ʢ��FeCl2��KSCN�Ļ��Һ������E��C�е������� ��C�з���������ԭ��Ӧ�����ӷ���ʽ�� ��
��2����A��ΪŨ���ᣬB��װ�й���KMnO4 ��C��ʢ��KI������Һ������E��B�г��ֻ���ɫ���塣��֪1mol KMnO4������Ӧʱת��5mol���ӣ������ɵ�����Ϊ���ʡ�B�з�����Ӧ�Ļ�ѧ����ʽ�� ��C�е������� ��������Ӧһ��ʱ�����C����Һ����ɫ��ȥ��������Ϊ����Һ�� ����ѧ����Ϊ����ʵ��װ�ò����ϻ���Ҫ����������Ľ������
��

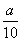 g��Ƭ��ȫ�ܽ��ڹ���ϡ�����У�����Ӧ��õ�����Һ��0.02000mol��L-1
g��Ƭ��ȫ�ܽ��ڹ���ϡ�����У�����Ӧ��õ�����Һ��0.02000mol��L-1
 2Z(g)һ���ﵽ��ѧƽ��״̬ (����)
2Z(g)һ���ﵽ��ѧƽ��״̬ (����)