��Ŀ����
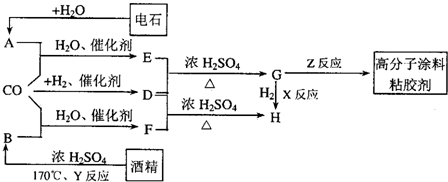
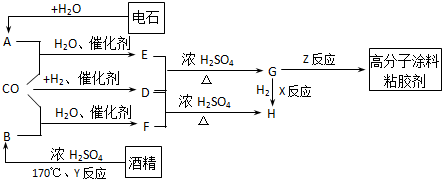
CO�����Ǽ���ú������Ҫ�ɷ֣�Ҳ����Ҫ�Ļ���ԭ�ϣ�����������������һ�ֵ��µ�ѹ�����գ���ijЩ���л��ᆳ���ʻ�����Ӧ�����������һ������������ܵ�װ���Ը߷���Ϳ�ϡ�ճ�ϼ��ȣ���ͼ��ʾ��
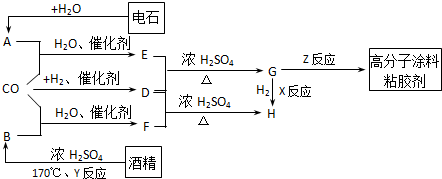
ͼ��G��R��COOR������һ��ͬ���칹����E������ͬϵ���H��һ��ͬ���칹������F������ͬϵ���֪D��CO��H2�����ʵ���֮��Ϊ1��2��ȫ��Ӧ���ɣ�����������ɷ���������Ӧ��H�Ǻ���4��̼ԭ�ӵĻ������д����
��1���ṹ��ʽ��E______��G______��R����______��
��2��G������ͬ���ͬ���칹��Ľṹ��ʽ������R��ĸ��______��______��
��3����Ӧ���ͣ�X______��Y______��Z______��
��4��д������ת���Ļ�ѧ����ʽ��
��A+CO+H2O
E�� ��F+D
H
��______����______��

ͼ��G��R��COOR������һ��ͬ���칹����E������ͬϵ���H��һ��ͬ���칹������F������ͬϵ���֪D��CO��H2�����ʵ���֮��Ϊ1��2��ȫ��Ӧ���ɣ�����������ɷ���������Ӧ��H�Ǻ���4��̼ԭ�ӵĻ������д����
��1���ṹ��ʽ��E______��G______��R����______��
��2��G������ͬ���ͬ���칹��Ľṹ��ʽ������R��ĸ��______��______��
��3����Ӧ���ͣ�X______��Y______��Z______��
��4��д������ת���Ļ�ѧ����ʽ��
��A+CO+H2O
| ���� |
| Ũ����� |
��______����______��
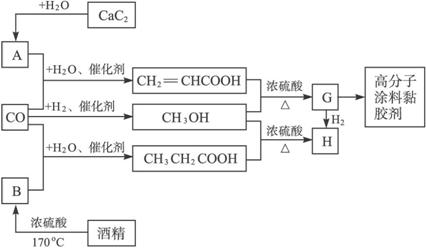
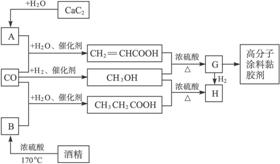
��ʯ��ˮ��Ӧ����A��AΪCH��CH��D��CO��H2�����ʵ���֮��Ϊ1��2��ȫ��Ӧ���ɣ�D�����ʽΪCH4O�����ʽ��Ϊ����ʽ������������ɷ���������Ӧ����DΪCH3OH��D��E��Ӧ����G��G����������EΪ���ᣬG�������������ӳɷ�Ӧ����H����H����4��̼ԭ�ӣ���G�к��в����ͼ�������4��Cԭ�ӣ���E����3��Cԭ�ӣ����CH��CH��CO��H2O����E����EΪCH2=CHCOOH��GΪCH2=CHCOOCH3��HΪCH3CH2COOCH3��F��D��CH3OH����Ӧ����H����F����-COOH���Һ�������Cԭ�ӣ���FΪCH3CH2COOH���ƾ���Ũ���ᡢ����170�������·�����ȥ��Ӧ����CH2=CH2����BΪCH2=CH2��CO��H2O����CH3CH2COOH��Gͨ���Ӿ۷�Ӧ���ɸ߷���Ϳ��ճ������
��1��������������֪��EΪCH2=CHCOOH��GΪCH2=CHCOOCH3��R��Ϊ-CH3��
�ʴ�Ϊ��CH2=CHCOOH��CH2=CHCOOCH3��-CH3��
��2��CH2=CHCOOCH3������ͬ���ͬ���칹��Ľṹ��ʽΪHCOOCH2CH=CH2��CH3COOCH=CH2��
�ʴ�Ϊ��HCOOCH2CH=CH2��CH3COOCH=CH2��
��3����Ӧ���ͣ�X���ڼӳɷ�Ӧ��Y������ȥ��Ӧ��Z���ڼӾ۷�Ӧ��
�ʴ�Ϊ���ӳɷ�Ӧ����ȥ��Ӧ���Ӿ۷�Ӧ��
��4��д������ת���Ļ�ѧ����ʽ��
��A+CO+H2O
E�� ��F+D
H
��Ӧ�ٵķ���ʽΪ��HC=CH+CO+H2O
H2C=CHCOOH��
�ʴ�Ϊ��HC=CH+CO+H2O
H2C=CHCOOH��
��Ӧ�ڵķ���ʽΪ��CH3CH2COOH+CH3OH
CH3CH2COOCH3+H2O��
�ʴ�Ϊ��CH3CH2COOH+CH3OH
CH3CH2COOCH3+H2O��
��1��������������֪��EΪCH2=CHCOOH��GΪCH2=CHCOOCH3��R��Ϊ-CH3��
�ʴ�Ϊ��CH2=CHCOOH��CH2=CHCOOCH3��-CH3��
��2��CH2=CHCOOCH3������ͬ���ͬ���칹��Ľṹ��ʽΪHCOOCH2CH=CH2��CH3COOCH=CH2��
�ʴ�Ϊ��HCOOCH2CH=CH2��CH3COOCH=CH2��
��3����Ӧ���ͣ�X���ڼӳɷ�Ӧ��Y������ȥ��Ӧ��Z���ڼӾ۷�Ӧ��
�ʴ�Ϊ���ӳɷ�Ӧ����ȥ��Ӧ���Ӿ۷�Ӧ��
��4��д������ת���Ļ�ѧ����ʽ��
��A+CO+H2O
| ���� |
| Ũ����� |
��Ӧ�ٵķ���ʽΪ��HC=CH+CO+H2O
| ���� |
�ʴ�Ϊ��HC=CH+CO+H2O
| ���� |
��Ӧ�ڵķ���ʽΪ��CH3CH2COOH+CH3OH
| Ũ���� |
| �� |
�ʴ�Ϊ��CH3CH2COOH+CH3OH
| Ũ���� |
| �� |

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
��14�֣�CO�����Ǽ���ú������Ҫ�ɷ֣�Ҳ����Ҫ�Ļ���ԭ�ϡ�����������������һ�ֵ��µ�ѹ�����գ���ijЩ���л��ᆳ���ʻ�����Ӧ�����������һ������������ܵ�װ���Ը߷���Ϳ�ϡ�ճ�ϼ��ȡ�����ͼ��ʾ��
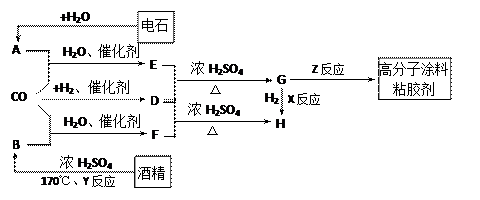 |
ͼ��G(RCOOR��)��һ��ͬ���칹����E������ͬϵ���H��һ��ͬ���칹������F������ͬϵ���֪D��CO��H2�����ʵ���֮��Ϊ1��2��ȫ��Ӧ���ɣ�����������ɷ���������Ӧ��H�Ǻ���4��̼ԭ�ӵĻ������д����
��1���ṹ��ʽ��E ��G ��R���� ��
��2��G������ͬ���ͬ���칹��Ľṹ��ʽ������R��ĸ�� �� ��
��3����Ӧ���ͣ�X ��Y ��Z ��
 ��4��д������ת���Ļ�ѧ����ʽ��
��4��д������ת���Ļ�ѧ����ʽ���� A + CO + H2O E�� ��F + D H
�� ��
�� ��