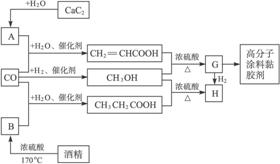
ĢāÄæÄŚČŻ
CO²»½öŹĒ¼ŅÓĆĆŗĘųµÄÖ÷ŅŖ³É·Ö£¬Ņ²ŹĒÖŲŅŖµÄ»Æ¹¤ŌĮĻ”£ĻĀĶ¼ŹĒÓĆijŠ©¼ņµ„ÓŠ»śĪļŌŚµĶĪĀ”¢µĶŃ¹ŗĶ“߻ƼĮ“ęŌŚĻĀŗĻ³É¾ßÓŠÓÅĮ¼ŠŌÄܵÄ×°ŹĪŠŌøß·Ö×ÓĶæĮĻ”¢š¤½ŗ¼ĮµÄ»ł±¾¹ż³Ģ”£
ŅŃÖŖCaC2ÓėĖ®·“Ó¦µÄ»Æѧ·½³ĢĪŖCaC2+2H2O![]() Ca(OH)2+HC”ŌCH”ü”£»Ų“šĻĀĮŠĪŹĢā£ŗ
Ca(OH)2+HC”ŌCH”ü”£»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)Š“³öGµÄ½į¹¹¼ņŹ½”£_____________________
(2)Š“³öĶ¼ÖŠÉś³ÉCH3OH(¼×“¼)µÄ»Æѧ·½³ĢŹ½”£Š“³öŌŚÅØĮņĖį×÷ÓĆĻĀÉś³ÉHµÄ»Æѧ·½³ĢŹ½”£_______________________________________________________________
(3)Öø³öÉĻŹö¹¤ŅÕµÄÓŵćŹĒŹ²Ć“£æ__________________________________________
½āĪö£ŗøł¾ŻGŹĒÓÉCH2=CHCOOHÓėCH3OHŌŚÅØĮņĖį×÷ÓĆĻĀ¼ÓČČ·“Ó¦µĆĄ“£¬ĒŅÄÜÓėH2·“Ó¦µĆH£¬æÉÖŖĘä½į¹¹¼ņŹ½ĪŖCH2=CHCOOCH3”£
“š°ø£ŗ(1)CH2=CHCOOCH3
(2)CO+2H2![]() CH3OH
CH3OH
CH3OH+CH3CH2COOH![]() CH3OOCCH2CH3
CH3OOCCH2CH3
(3)ŌĮĻ³É±¾µĶ£¬·“Ó¦ŌŚµĶĪĀ”¢µĶŃ¹ŗĶ“߻ƼĮĢõ¼žĻĀ½ųŠŠ£¬ÄÜĮæĻūŗĵĶ£¬·“Ó¦¹ż³Ģ֊ƻӊČĪŗĪø±²śĘ·²śÉś£¬ŌĮĻĄūĀŹøß”£

£Ø14·Ö£©CO²»½öŹĒ¼ŅÓĆĆŗĘųµÄÖ÷ŅŖ³É·Ö£¬Ņ²ŹĒÖŲŅŖµÄ»Æ¹¤ŌĮĻ”£ĆĄ¹ś½üÄźĄ“±Øµ¼ĮĖŅ»ÖÖµĶĪĀµĶŃ¹“߻ƹ¤ŅÕ£¬°ŃijŠ©¼ņµ„µÄÓŠ»śĪļ¾”°ōŹ»Æ”±·“Ó¦ŗóæÉŅŌ×īŗó²śÉśŅ»Ąą¾ßÓŠÓÅĮ¼ŠŌÄܵÄ×°ŹĪŠŌøß·Ö×ÓĶæĮĻ”¢Õ³ŗĻ¼ĮµČ”£ČēĻĀĶ¼ĖłŹ¾£ŗ
 |
Ķ¼ÖŠG(RCOOR£¬)µÄŅ»ÖÖĶ¬·ÖŅģ¹¹ĢåŹĒEµÄĻąĮŚĶ¬ĻµĪļ£»¶ųHµÄŅ»ÖÖĶ¬·ÖŅģ¹¹ĢåŌņŹĒFµÄĻąĮŚĶ¬ĻµĪļ”£ŅŃÖŖDÓÉCOŗĶH2°“ĪļÖŹµÄĮæÖ®±ČĪŖ1”Ć2ĶźČ«·“Ó¦¶ų³É£¬ĘäŃõ»Æ²śĪļæÉ·¢ÉśŅų¾µ·“Ó¦£»HŹĒŗ¬ÓŠ4øöĢ¼Ō×ӵĻÆŗĻĪļ”£ŹŌŠ“³ö£ŗ
£Ø1£©½į¹¹¼ņŹ½£ŗE ”¢G ”¢R£¬»ł ”£
£Ø2£©GµÄĮ½øöĶ¬Ąą±šĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½£Ø²»“ųR×ÖÄø£© ¼° ”£
£Ø3£©·“Ó¦ĄąŠĶ£ŗX ”¢Y ”¢Z ”£

 £Ø4£©Š“³öĻĀĮŠ×Ŗ»ÆµÄ»Æѧ·½³ĢŹ½£ŗ
£Ø4£©Š“³öĻĀĮŠ×Ŗ»ÆµÄ»Æѧ·½³ĢŹ½£ŗ¢Ł A + CO + H2O E£» ¢ŚF + D H
¢Ł £»
¢Ś ”£