��Ŀ����
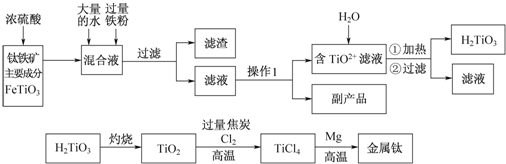
6��Ϊ�о�ͭ��Ũ����ķ�Ӧ��ij��ѧ��ȤС���������ʵ�飮ʵ��I����Ӧ����Ķ���̽��������ͼװ�ã��̶�װ������ȥ������ʵ�飺
��1��Fװ�õ��ձ��з�����Ӧ�����ӷ���ʽ��SO2+2OH-=SO32-+H2O��Bװ���е������ǰ�ɫ��ĩ����ɫ��
��2��ʵ������У���֤��Ũ��������Ԫ�ص�������ǿ����Ԫ�ص�������Dװ���к�ɫ������ɫ�ޱ仯��Eװ������Һ��ɫ
��3��ʵ�����ʱ����ȥ���оƾ���֮ǰ������ɵ�ʵ�����������ͭ˿���ر�K1��K2
��4��ʵ�������֤��Aװ���Թ��з�Ӧ���ò����Ƿ���ͭ���ӵIJ��������ǽ�Aװ������ȴ�Ļ����Һ���ձ��ڱڻ�������ʢˮ���ձ��У������Ͻ��裬�۲��Ƿ�����ɫ����
ʵ���Ӧ����Ķ���̽��
��5����ͭ��Ũ���ᷴӦ�Ĺ����У������к�ɫ���ʳ��֣��Һ�ɫ����ΪCu2S������Cu2S�ķ�ӦΪaCu+bH2SO4$\frac{\underline{\;\;��\;\;}}{\;}$cCu2S+dCuSO4+e H2O����a��b=5��4��
��6��Ϊ�ⶨ����ͭ�IJ��ʣ����÷�Ӧ������Һ�кͺ����Ƴ�250.00mL��Һ��ȡ����Һ25.00mL��������KI��Һ�����Ե�����ҺΪָʾ������O��36mol•L-1��Na2S2O3��Һ�ζ����ɵ�I2��3��ʵ��ƽ�����ĸ�Na2S2O3��Һ25.00mL������Ӧ����ͭ������Ϊ6.4g��������ͭ�IJ���Ϊ90%��
����֪2Cu2++4I-=2CuI+I2��2S2O32-+I2=S4O62-+2I-��
���� ���������£�Cu��Ũ���ᷢ��������ԭ��Ӧ��������ͭ�����������ˮ��ˮ���лӷ��ԣ��¶�Խ�ӷ���Խǿ���������ɵĶ��������к���ˮ������ˮ������ʹ��ˮ����ͭ����ɫ��Ũ���������ˮ�ԣ��ܸ�������������壻�������л�ԭ�ԣ��ܽ���ɫ������ͭ��ĩ��ԭΪ��ɫCu�������������Ư���ԣ���ʹƷ����Һ��ɫ���������������������������ж�������ֱ���ſգ������ü�����Һ���գ�
��1�����������NaOH��Ӧ�����������ƺ�ˮ����ˮ����ͭ����ˮ������
��2��ʵ������У�Ҫ֤��Ũ��������Ԫ�ص�������ǿ����Ԫ�أ�ֻҪ֤�����ɵ������к��ж��������������ɣ�
��3��ʵ�����ʱ����ȥ���оƾ���֮ǰҪ��ֹ������
��4��ͭ������ˮ��Һ�г���ɫ��������Һ��ɫ�ж��Ƿ���ͭ���ӣ�
��5������ת�Ƶ��Ӽ�ԭ���غ���ƽ����ʽ���Ӷ�ȷ��a��b��ϵ��
��6������2Cu2++4I-=2CuI+I2��S2O32-+I2=S4O62-+2I-��2Cu2+��I2��S2O32-������ͭ���Ӻ�������������֮��Ĺ�ϵʽ���㣮
��� �⣺���������£�Cu��Ũ���ᷢ��������ԭ��Ӧ��������ͭ�����������ˮ��ˮ���лӷ��ԣ��¶�Խ�ӷ���Խǿ���������ɵĶ��������к���ˮ������ˮ������ʹ��ˮ����ͭ����ɫ��Ũ���������ˮ�ԣ��ܸ�������������壻�������л�ԭ�ԣ��ܽ���ɫ������ͭ��ĩ��ԭΪ��ɫCu�������������Ư���ԣ���ʹƷ����Һ��ɫ���������������������������ж�������ֱ���ſգ������ü�����Һ���գ�
��1�������������������������NaOH��Ӧ�����������ƺ�ˮ�����ӷ�Ӧ����ʽΪ SO2+2OH-=SO32-+H2O��ˮ���лӷ��ԣ������¶ȴٽ���ӷ����������ɵ������к���ˮ������ˮ������ʹ��ˮ����ͭ�ɰ�ɫ��Ϊ��ɫ������Bװ���е������ǣ���ɫ��ĩ����ɫ��
�ʴ�Ϊ��SO2+2OH-=SO32-+H2O����ɫ��ĩ����ɫ��
��2��֤��Ũ��������Ԫ�ص�������ǿ����Ԫ�أ�����Ԫ�ػ��ϼ۱仯���ɵIJ�������жϣ�������������Dװ�û��ɫ�仯Ϊ��ɫ�������ɶ�������Eװ����Ʒ�����ɫ������֤��Ũ��������Ԫ�ص�������ǿ����Ԫ�ص�ʵ�������ǣ�Dװ��������ͭ��ɫ���仯��˵�����������ɣ�Eװ����Ʒ����Һ��ɫ˵�������˶����������壬
�ʴ�Ϊ��Dװ���к�ɫ������ɫ�ޱ仯��Eװ������Һ��ɫ��
��3��ʵ�����ʱ������ͭ˿����ȥ���оƾ���֮ǰ������ɵ�ʵ������ǹر�K1��K2����ֹCE�е���Һ������ը�Ѳ����ܣ��ʴ�Ϊ������ͭ˿���ر�K1��K2��
��4����֤���ɵ���Һ�к���ͭ���ӣ���Ҫ���Թ��е�Һ�嵹��ˮ���ܽ⣬�۲��Ƿ������ɫ��Һ����Aװ������ȴ�Ļ����Һ���ձ��ڱڻ�������ʢˮ���ձ��У������Ͻ��裬������ɫ��Һ֤������ͭ���ӣ�
�ʴ�Ϊ����Aװ������ȴ�Ļ����Һ���ձ��ڱڻ�������ʢˮ���ձ��У������Ͻ��裬�۲��Ƿ�����ɫ���֣�
��5��aCu+bH2SO4$\frac{\underline{\;\;��\;\;}}{\;}$cCu2S+dCuSO4+e H2O��CuԪ�ػ��ϼ���0�۱�Ϊ+1�ۡ�+2�ۡ�SԪ�ػ��ϼ���+6�۱�Ϊ-2�ۣ�ת�Ƶ�����Ϊ6������ת�Ƶ����غ㼰ԭ���غ���ƽ����ʽΪ5Cu+4H2SO4$\frac{\underline{\;\;��\;\;}}{\;}$Cu2S+3CuSO4+4H2O������a��b=5��4��
�ʴ�Ϊ��5��4��
��6������2Cu2++4I-=2CuI+I2��2S2O32-+I2=S4O62-+2I-��2Cu2+��I2��2S2O32-��
���Ƶõ���Һ������ͭ�����ʵ���Ũ��Ϊxmol/L��
2Cu2+��I2��2S2O32-��
2mol 2mol
0.025xmol ��0.36��0.025��mol
2molL��2mol=0.025xmol����0.36��0.025��mol
x=$\frac{2mol����0.36��0.025��mol}{0.025mol��2mol}$=0.36��
��250mL��Һ��n��Cu2+��=0.36mol/L��0.25L=0.09mol���μӷ�Ӧ��n��Cu��=$\frac{6.4g}{64g/mol}$=0.1mol��
������ͭ�IJ���=$\frac{0.09mol}{0.1mol}��100%$=90%��
�ʴ�Ϊ��90%��
���� ������Ũ�����ͭ�ķ�ӦΪ���忼������ʵ�鷽����ƣ��漰������ȡ��������顢��Ӧ����ʽ����ƽ������ʽ�ļ����֪ʶ�㣬�ۺ��Խ�ǿ����ȷ��Ӧԭ�������������ǽⱾ��ؼ���֪���������������ļ��鷽����˳����Ŀ�Ѷ��еȣ�

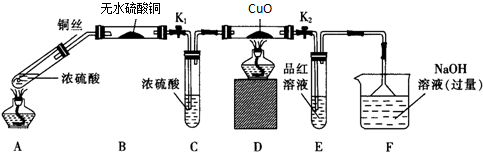
| A�� | HCl | B�� | CO2 | C�� | NH3 | D�� | SO2 |
| ʵ�� װ�� |  |  |  | 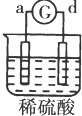 |
| ����ʵ������ | a��������Сb���������� | c����������� b���������� | d���ܽ�c����������� | ������a������d�� |
| A�� | a��b��c��d | B�� | b��c��d��a | C�� | d��a��b��c | D�� | a��b��d��c |
| A�� | �ڽ����к͵ζ�ʱ���ñ�����Һ��ϴ��ʽ�ζ���2��3�Σ�������ˮϴ2��3�Σ�Ч������ | |
| B�� | ���������������ϡ���ᷴӦ����S��SO2���ڶ��������ⶨ��Ӧ����ʱ��������S�Ա���ڸǷ���Ҳ������ˮ����SO2������������صķ�Ӧ���� | |
| C�� | ȼ�ϵ�ص��������ð��б����������̼�����缫���NaSO4��Һ��һ��ʱ����жϵ�Դ��������֮����Ϸ�������ܣ����ֶ����ܷ��� | |
| D�� | ���ˡ��ᾧ�����ա���ȡ����Һ������ȶ��dz��õķ�������ķ��� |
| A�� | ԭ��Һ�϶�ֻ����NH4+��SO32- | |
| B�� | ԭ��Һһ������Ba2+��Fe3+��I- | |
| C�� | ԭ��Һ���ܴ���K+��SO42- | |
| D�� | ��ȡ��Һ�μ��������ᡢBaCl2��Һ������ȷ����Һ������� |
| A�� | 71gNa2SO4�����к���������ĿΪNA | |
| B�� | 28g����ϩ�ͱ�ϩ��ɵĻ�������к�̼̼˫����ĿΪNA | |
| C�� | ��״���£�22.4LHF�к�HF������ĿΪNA | |
| D�� | 25g��������Ϊ68%��H2O2ˮ��Һ�к���ԭ����ĿΪNA |
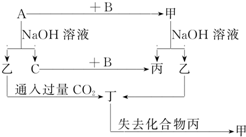 �ɶ�����Ԫ����ɵĵ���A��B��C�ͼס��ҡ����������ֻ�������ͼʾ��ת����ϵ����֪CΪ�ܶ���С�����壮
�ɶ�����Ԫ����ɵĵ���A��B��C�ͼס��ҡ����������ֻ�������ͼʾ��ת����ϵ����֪CΪ�ܶ���С�����壮