��Ŀ����
�����£���25 mL 0.1 mol��LNaOH��Һ����μ���0.2 mol��L CH3COOH��Һ����������ͼ��ʾ������������Һ���ʱ������仯���й�����Ũ�ȹ�ϵ�Ƚϴ������
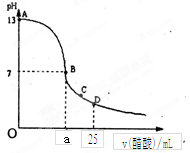
A����A��B����һ�㣬��Һ��һ�����У�c(Na+)+c(H+)= c(CH3COO��)+c(OH��)
B����B�㣺a>12.5������c(Na+)=c(CH3COO��)=c(OH��)=c(H+)
C����C�㣺c(Na+)>c(CH3COO��) >c(H+)>c(OH��)
D����D�㣺c(CH3COO��)+c(CH3COOH)��0.1mol/L

A����A��B����һ�㣬��Һ��һ�����У�c(Na+)+c(H+)= c(CH3COO��)+c(OH��)
B����B�㣺a>12.5������c(Na+)=c(CH3COO��)=c(OH��)=c(H+)
C����C�㣺c(Na+)>c(CH3COO��) >c(H+)>c(OH��)
D����D�㣺c(CH3COO��)+c(CH3COOH)��0.1mol/L
BC
��������25mL 0.1mol��L-1NaOH��Һ����μ���0.2mol?L-1 CH3COOH ��Һ������֮�����Ӧ����ǡ����ȫ��Ӧʱ�������������Ϊ12.5mL������Ӧ����Һ������ʱ������Ӧ�Թ�������c��OH-��=c��H+����ע����ݵ���غ�˼�����Ƚ�����Ũ�ȴ�С��
�⣺A����A��B����һ�㣬��Һ��ֻ��������������Na+��H+��CH3COO-��OH-�����ݵ���غ����У�
c��Na+��+c��H+��=c��CH3COO-��+c��OH-������A��ȷ��
B����B����Һ�����ԣ�������c��OH-��=c��H+�������ݵ���غ�c��Na+��+c��H+��=c��CH3COO-��+c��OH-������һ����
c��Na+��=c��CH3COO-������Һ�ijɷ�Ϊ����Ӧ���ɵĴ����ƺ�ʣ��Ĵ��ᣬ�����Ƶ�ˮ��̶Ⱥʹ���ĵ���̶���ȣ����У�c��Na+��=c��CH3COO-����c��OH-��=c��H+������B����
C����C�㣬��Һ�����ԣ�����c��OH-����c��H+�������ݵ���غ㣺c��Na+��+c��H+��=c��CH3COO-��+c��OH-������c��Na+����c��CH3COO-������C����
D����D��ʱ������ʣ�࣬ʣ��Ĵ����Ũ�Ⱥ����ɵĴ�����Ũ����Ⱦ�Ϊ0.05mol/l�����������غ㣬��
c��CH3COO-��+c��CH3COOH��=0.1mol?L-1����D��ȷ��
��ѡBC��

��ϰ��ϵ�д�
�����Ŀ
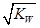
 0.05mol��
0.05mol�� ��ѡ����ţ� ��
��ѡ����ţ� �� ��<������=������ͬ�� c��NH
��<������=������ͬ�� c��NH 3��H2O���� ��Ϻ���Һ��c��NH4+����c��Cl�����Ĺ�ϵc��NH4+�� c��Cl����
3��H2O���� ��Ϻ���Һ��c��NH4+����c��Cl�����Ĺ�ϵc��NH4+�� c��Cl����  H3COOH
H3COOH  .CH3COOH��NaOH�Ļ����Һ�Լ��ԣ�����Һ�и�����Ũ�ȴ�С����һ��Ϊ
.CH3COOH��NaOH�Ļ����Һ�Լ��ԣ�����Һ�и�����Ũ�ȴ�С����һ��Ϊ