��Ŀ����
(13��)A��һ�־��ô��ᵯ����Ҫ�ɷ֡�������A����Է�������Ϊ161��������C��HԪ���⣬��������һ��±��Ԫ�أ�������ֻ����һ������������A��H��ת����ϵ����ͼ��ʾ����������������Cu(OH)2����Һ��1 mol C��Ӧ������1 mol Cu2O ��1 mol D��B1��B2��Ϊͬ���칹�壬B1��Ħ������80g/mol��G1��G2��Ϊͬ���칹�壬���ߵĺ˴Ź�������ֻ�����������G1����

��һ��̼ԭ������������̼̼˫���Ľṹ(-C=C=C-)���ȶ���
�������������
(1)��A�����ŵ�����
(2)��~��Ӧ��������ȥ��Ӧ����_________��A�Ľṹ��ʽ��__________________
(3)��Ӧ�ܵĻ�ѧ����ʽ��________________________
(4)д��C������Cu(OH)2��Ӧ�ķ���ʽ��____________________
(5)һ��������H�ܹ����ɸ߷��ӻ����д����Ӧ�ķ���ʽ
(6)��������������E��ͬ���칹�干��________��;
�ٺ�����������������NaHCO3��Ӧ����-OH��-Br������ͬһ��̼ԭ���ϡ�

��һ��̼ԭ������������̼̼˫���Ľṹ(-C=C=C-)���ȶ���
�������������
(1)��A�����ŵ�����
(2)��~��Ӧ��������ȥ��Ӧ����_________��A�Ľṹ��ʽ��__________________
(3)��Ӧ�ܵĻ�ѧ����ʽ��________________________
(4)д��C������Cu(OH)2��Ӧ�ķ���ʽ��____________________
(5)һ��������H�ܹ����ɸ߷��ӻ����д����Ӧ�ķ���ʽ
(6)��������������E��ͬ���칹�干��________��;
�ٺ�����������������NaHCO3��Ӧ����-OH��-Br������ͬһ��̼ԭ���ϡ�
(1)̼̼˫������ԭ��(2) ��
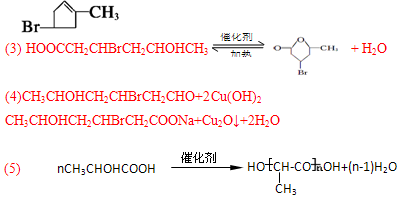
(6)2

(6)2
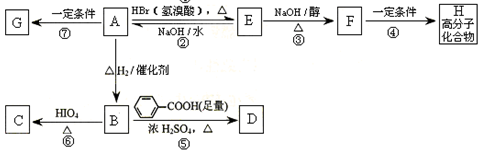
��F��֪A�к���6��̼ԭ�Ӻ�1����ԭ�ӣ���˺��е���ԭ����161��72��80��9��������ʽΪC6H9Br��6��̼ԭ�������14����ԭ�ӣ����A�п�����1��̼̼������2��̼̼˫����1��̼̼˫����1��̼��������A����������ֻ��C�����Ը�����Ϣ�ٿ�֪A��Ӧ�ú���1��̼̼˫����1��̼����1 mol C��Ӧ������1 mol Cu2O��˵��ֻ����1��ȩ��������һ�������͵�̼ԭ���������ʻ�����˼������ڲ�����̼ԭ���ϡ�C��������D��ȩ�������Ȼ���D�ӳ�����E���ʻ����ǻ�������E�м����Ȼ��������ǻ���ͨ��������Ӧ����F��Aͨ����ȥ��Ӧ����B1��B2������䷴���к���2��̼̼˫����B2ͨ����Ӧ������G1��G2�����Է����ں����ʻ���ȩ����G1��G2��Ϊͬ���칹�壬��˸�����3��̼ԭ�ӣ�����Ϊ���ߵĺ˴Ź�������ֻ�����������G1��������G1��G2�Ľṹ��ʽ�ֱ�ΪOHCCH2CHO��CH3COCHO����H�Ľṹ��ʽ��CH3CHOHCOOH������H�����ں����ǻ����Ȼ�����ͨ�����۷�Ӧ���ɸ߷��ӻ����������Ϣ�٢ڿ���B2�Ľṹ��ʽΪ
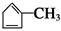 ��B1�ýṹ��ʽ��
��B1�ýṹ��ʽ�� ����A�ýṹ��ʽ��
����A�ýṹ��ʽ��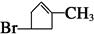 ���ɴ˵ó�CDEF�ýṹ��ʽ�ֱ�ΪOHCCH2CHBrCH2COCH3��HOOCCH2CHBrCH2COCH3��CH3CHOHCH2CHBrCH2COOH��
���ɴ˵ó�CDEF�ýṹ��ʽ�ֱ�ΪOHCCH2CHBrCH2COCH3��HOOCCH2CHBrCH2COCH3��CH3CHOHCH2CHBrCH2COOH��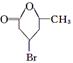 ������E��ͬ���칹����(CH3)2CBrC(CH3)OHCOOH��(CH3)2COHC(CH3)BrCOOH��
������E��ͬ���칹����(CH3)2CBrC(CH3)OHCOOH��(CH3)2COHC(CH3)BrCOOH��
 ��B1�ýṹ��ʽ��
��B1�ýṹ��ʽ�� ����A�ýṹ��ʽ��
����A�ýṹ��ʽ�� ���ɴ˵ó�CDEF�ýṹ��ʽ�ֱ�ΪOHCCH2CHBrCH2COCH3��HOOCCH2CHBrCH2COCH3��CH3CHOHCH2CHBrCH2COOH��
���ɴ˵ó�CDEF�ýṹ��ʽ�ֱ�ΪOHCCH2CHBrCH2COCH3��HOOCCH2CHBrCH2COCH3��CH3CHOHCH2CHBrCH2COOH�� ������E��ͬ���칹����(CH3)2CBrC(CH3)OHCOOH��(CH3)2COHC(CH3)BrCOOH��
������E��ͬ���칹����(CH3)2CBrC(CH3)OHCOOH��(CH3)2COHC(CH3)BrCOOH��
��ϰ��ϵ�д�
�����Ŀ
 ��������ˮ���ǹ������Ӽ�;
��������ˮ���ǹ������Ӽ�; 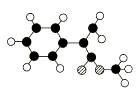
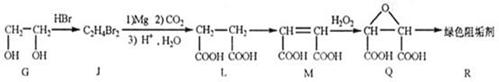
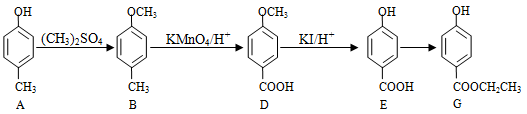
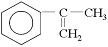 Ϊ��Ҫԭ�Ϻϳ�A��·�����£�
Ϊ��Ҫԭ�Ϻϳ�A��·�����£�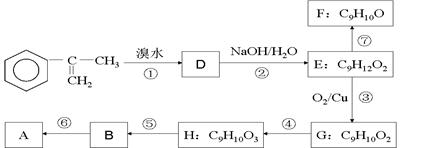
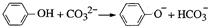
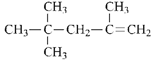 ������Ϊ��2,2,4-����-4-��ϩ
������Ϊ��2,2,4-����-4-��ϩ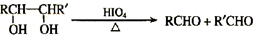
 ��ɫ��ԭ���� ��������A��ˮ�е��ܽ�ȱȱ��ӵ� �����С������
��ɫ��ԭ���� ��������A��ˮ�е��ܽ�ȱȱ��ӵ� �����С������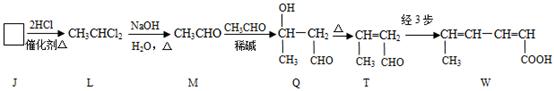
 ��
�� Q
Q Һ�� D��������
Һ�� D��������
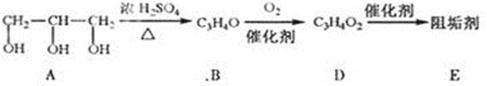
 _ˮ���Ƶá�(����ࡱ������֬�������ʡ�)
_ˮ���Ƶá�(����ࡱ������֬�������ʡ�) ___
___