��Ŀ����
�����12�֣�
������Ȼ����������̬�Ͷ��ֻ���̬�γɳ��֡���Ļ���������������Ի�ԭ�ԡ������������������ˮ��
���������գ�
(1)������л�ԭ�ԣ����Ժ�������������Ӧ�������������£�H2S��KMnO4��Ӧ����S��MnSO4��K2SO4��H2O��д���÷�Ӧ�Ļ�ѧ����ʽ��_________________________________
(2)ʯ�ͻ����ķ�������H2S��д���ӷ����л��յ���������ַ������������⣬��ʹ������ԭ�ϣ����Ի�ѧ����ʽ��ʾ��_____________________��______________________
(3)�����£�0.1mol/L��������Һ��0.1mol.L��̼������Һ�����Ը�ǿ����_______����ԭ����________��
��֪��H2S��Ki1��1.3��10��7 Ki2��7.1��10��15
H2CO3��Ki1��4.3��10��7 Ki2��5.6��10��11
(4)��ZnSO4��Һ�еμӱ���H2S��Һ��û�г������ɣ������μ�һ�����İ�ˮ������ZnS�������õ���ƽ��ԭ��������������__________________________
(5)����ɫ��Fe2S3����������������У���Һ���е���ɫ�������ɣ����ﻹ��____��______�����ˣ�����Һ��Ȼ��������������������Һ���ɹ۲쵽��������______________��
(1)5H2S��2KMnO4��3H2SO4��K2SO4��2MnSO4��8H2O��5S��
(2)2H2S��3O2��ȼ2SO2��2H2O��2H2S��2SO2��3S��2H2O��H2S��S��H2��2H2S��O2��ȼ2S��2H2O
(3)������Һ�������Ki2С��̼���Ki2�����Ƹ���ˮ�⡣
(4)����H2S��Һ�е��������S2�����٣����û�г��������백ˮ�ٽ�H2S�ĵ��룬S2������Ũ�������г���������
(5)FeCl2��H2S�����а�ɫ�������ɣ�Ȼ�����ת��Ϊ����ɫ������ת��Ϊ���ɫ��
�������������(1)���ݷ�Ӧ�������������������غ㶨�ɺ͵��ӵ�ʧ�غ��֪��Ӧ�Ļ�ѧ����ʽΪ5H2S��2KMnO4��3H2SO4��K2SO4��2MnSO4��8H2O��5S����
(2)H2S����ȫȼ�տ������ɵ���������H2S�ֽ�Ҳ���ɵ���������SO2Ҳ����H2S��Ӧ���ɵ�����H2S��ȫȼ�ռ����� SO2������йصķ�Ӧ�Ļ�ѧ����ʽΪ2H2S��3O2��ȼ2SO2��2H2O��2H2S��2SO2��3S��2H2O��H2S��S��H2��2H2S��O2��ȼ2S��2H2O��
(3)��Խ������Ӧ������Խ����ˮ�⣬��Һ�ļ���Խǿ�����ݵ��볣����֪�������Ki2С��̼���Ki2�����Ƹ���ˮ�⣬��������Һ�ļ��Ը�ǿ��
(4)H2S��������ʣ����ڵ���ƽ�⡣����H2S��Һ�е��������S2�����٣����û�г��������백ˮ�ٽ�H2S�ĵ��룬S2������Ũ�����Ӷ�����������
(5)����ɫ��Fe2S3����������������У���Һ���е���ɫ�������ɣ��ó����ǵ�������˵��������������ԭ��Ӧ�������ӽ��������������ɵ�������������ԭ�����������ӡ����������ӹ������������������������ӽ������H2S���壬������FeCl2��H2S���ɣ����Ȼ�������Һ�м�������������Һ����������������ɫ�������������������ȶ�Ѹ�ٱ���������������������˹۲쵽��ʵ�����������а�ɫ�������ɣ�Ȼ�����ת��Ϊ����ɫ������ת��Ϊ���ɫ��
���㣺����H2S�����ʡ�������ԭ��Ӧ�����Ļ������Լ�����ƽ����ܽ�ƽ���Ӧ��

 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д���ѧ�ҷ�����������Ե�����Ѫ�ܹ��ܡ��й������������ȷ����
| A��������ȶ��������ѷֽ� |
| B��������ͨ����ˮ�У���ˮ��ɫ���������� |
| C������������������ȼ�����ɵ���ɫ���� |
| D��������������ʣ�����뷽��ʽ��H2S ? 2H+ + S2- |
�����й������������в���ȷ���ǣ� ��
| A��������һ�ֻ���ɫ���д̼�����ζ������ |
| B��������Һ�ȣ���ˮ��ͬһ������ |
| C������������ˮ |
| D��������һ���ж����� |
(17��)Na2SO3�����ڿ����о������ױ��� ��
(I)Na2SO3�����ڿ����б��ʵ���Ҫԭ���û�ѧ����ʽ��ʾΪ ��
(II)Ϊ̽��Na2SO3��Ʒ�ı����������������¼��裺
����1��Na2SO3��Ʒ��ȫ���ʣ� ����2��Na2SO3��Ʒ��ȫû�б��ʣ�����3�� ��
�����������ʵ����̼�����ͽ��ۣ����̽����
| ʵ����� | ����ͽ��� |
| ����1��ȡ������Ʒ���Թ��У�������������ˮ����ܽ⣬�ٵμ�H2SO4�ữ��KMnO4��Һ�� | ������KMnO4��Һ���Ϻ�ɫ��Ϊ��ɫ �ٽ��ۣ���Ʒ���� ���ӣ�����1�������� ����ɫ�����ӷ���ʽΪ�� �� |
| ����2����ȡ������Ʒ���Թ��У�������������ˮ����ܽ⣬�ٵμ�ϡHCl��ʹ��Һ�����ԣ��ٵμ�����BaCl2��Һ�� | �������� �� ���ۣ�����2������ |
| ���� | ���� |
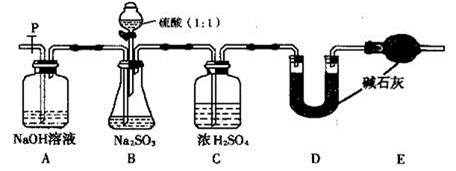
���Bװ�������ԵIJ���Ϊ ��
��2��ʵ���д���ƿ�в��ٲ����������P�ӵ�����˻�������һ�����Ŀ�������������Ŀ���� ��
��3�����ѳ�����a g Na2SO3��Ʒ�⣬ʵ���л�Ӧ�ⶨ�������� ��(��ͼ����ĸ)װ��ʵ��ǰ��������
��13�֣����仯�����й㷺��Ӧ�ã���SO2���ʵ��о��Ǹ��л�ѧ��ѧ��һ����Ҫ���ݡ�
��1���Ա��о���һ����Ҫ���о�������������ĵ��ʼ����ֻ����ﰴ���±���ʾ�ֳ�3�飬���2��������M�Ļ�ѧʽ�� ��
| ��1�� | ��2�� | ��3�� |
| S�����ʣ� | SO2��H2SO3��M��NaHSO3 | SO3��H2SO4����Na2SO4��NaHSO4 |
��2��ijУ��ѧѧϰС������ͼ��ʾ��ʵ��װ���о�SO2�����ʡ�
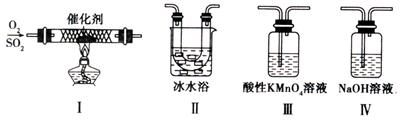
����װ�â��з���SO2�Ĵ�������Ӧ���仯ѧ����ʽ�� ��
����������˳������װ�ã�����֪��SO3�۵� 16.8�棻 SO2�е㡪10�档��װ�â�������� ��װ�â�����Һ����ɫ������Mn2+��ͬʱpH���ͣ���÷�Ӧ�����ӷ���ʽ�� ��
����������˳������װ�ã����װ�â�����30mL 2.5mol/LNaOH��Һ����Ӧ������4.8g����װ�â��з�����Ӧ�Ļ�ѧ����ʽ�� ��