��Ŀ����
��12�֣���1�� 0.3mol����̬����ȼ�������飨B2H6����������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�649.5ǧ�����������Ȼ�ѧ����ʽ�ǣ�
��
����֪��H2O��Һ����H2O����������H =-44kJ/mol����11.2������״���£���������ȫȼ��ʱ������̬ˮʱ�ų�������Ϊ ǧ����
��2����25�桢101kPa�£�1g�״���CH3OH��ȼ������CO2��Һ̬ˮʱ����22.68kJ�����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪ________________ ________________��
��3����֪��1mol H��H����1molN��H����1molN��N���ֱ���Ҫ��������436kJ��391kJ��946kJ����N2��H2��Ӧ����NH3���Ȼ�ѧ����ʽΪ________________________________��
��12�֣�
��1�� B2H6(g)+3O2(g)��B2O3(s)+3H2O(l) ��H���C2165.0kJ��mol��1�� 1016.5
��2��CH3OH(l)+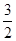 O2(g)��CO2(g)+2H2O(l)
��H���C725.76kJ��mol��1
O2(g)��CO2(g)+2H2O(l)
��H���C725.76kJ��mol��1
��3��N2(g)+3H2(g) === 2NH3(g) ��H����92kJ��mol��1
����������

 ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�| A��ZΪ0.3mol��L-1�� | B��X2Ϊ0.2mol��L-1�� | C��Y2Ϊ0.3mol��L-1�� | D��ZΪ0.4mol��L-1�� |