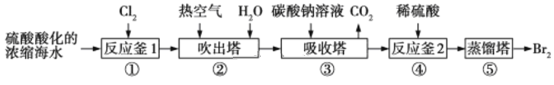
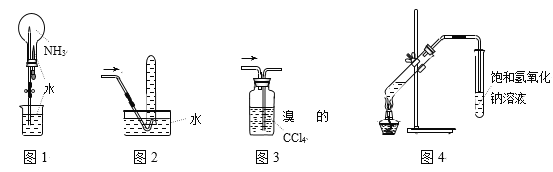
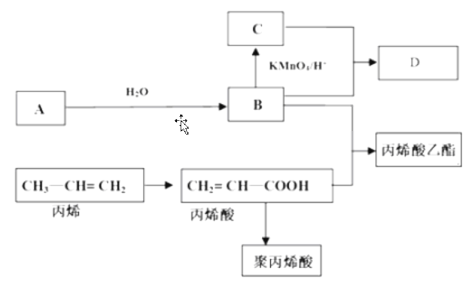
��Ŀ����
����Ŀ����֪���� CH3COOH(l)��2O2(g)��2CO2(g)��2H2O(l)������H1��a kJ��mol��1
�� C(s)��O2(g)��CO2(g) ������H2��b kJ��mol��1
�� 2H2O(l)��2H2(g)��O2(g) ������H3��c kJ��mol��1
�� 2C(s)��2H2(g)��O2(g)��CH3COOH(l) ������H4��d kJ��mol��1 ����˵����ȷ����
A. ʹ�ô�������H1��С B. b��0
C. H2(g)��![]() O2(g)��H2O(l) ��H��
O2(g)��H2O(l) ��H��![]() kJ��mol��1 D. d��2b��c��a
kJ��mol��1 D. d��2b��c��a
���𰸡�D
��������
A.��H�����������
B.��Ϊ���ȷ�Ӧ��b<0��
C. ��H��- c/2 kJ��mol��1��
D.���ø�˹���ɼ��㷴Ӧ����H��
A.ʹ�ô����ܹ��ı䷴Ӧ�Ļ�ܣ������ı���H����A�����
B.��Ϊȼ�շ�Ӧ����Ӧ���ȣ�b<0��
C.2H2O(l)��2H2(g)��O2(g)����H3��c kJ��mol��1�ɸ�˹���ɿ�֪��H2(g)��1/2O2(g)��H2O(l) ��H��- c/2 kJ��mol��1����C�����
D.���ݸ�˹���ɣ�����2-��-�ٵõ�������H4��d kJ��mol��1 =(2b��c��a) kJ��mol��1����D����ȷ��
���ϣ�����ѡD��

 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�����Ŀ���±���Ԫ�����ڱ���ǰ�����ڣ���ش��й����⣺����ؾ��þ��廯ѧ����ش�
1 | �� | |||||||
2 | �� | �� | �� | |||||
3 | �� | �� | �� | �� | ||||
4 | �� | �� | ||||||
��1��Ԫ�آݢޢߵļ����Ӱ뾶��С˳��__________������Ԫ�����ڱ�λ��________���۵ļ��⻯��͢���⻯�����γɵĻ��������ʽ__________��
��2���������������ˮ����͢���������ﷴӦ�����ӷ���ʽ_______
��3���õ���ʽ��ʾԪ�آٺ͢��γɵĻ�������γɹ���__________��
��4���ܡ��ࡢ����⻯��е���ߵ���__________��ԭ����__________��
��5���ɢں͢�������������O2�͢������������Ӧ��ˮ�����ˮ��Һ���ȼ�ϵ�أ�д����صĸ�����Ӧʽ__________��