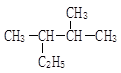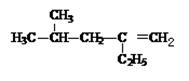��Ŀ����
��15�֣�����ѧ����ѡ��5���л���ѧ������
��֪����һ��̼ԭ�������������ǻ�ʱ����������ת������R��R�������������ԭ�ӣ���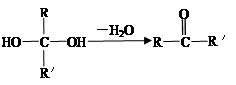
��ͬһ��̼ԭ������������˫���Ľṹ���ȶ���������ͼ�ش��й����⣺
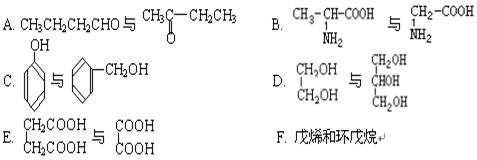
��1��E�к��еĹ����ŵ������� ��C������������ͭ��Ӧ�Ļ�ѧ����ʽΪ ��
��2��A�Ľṹ��ʽΪ ��A���ܷ����ķ�Ӧ�� ������ĸ����
a��ȡ����Ӧ b����ȥ��Ӧ c��������Ӧ d����ԭ��Ӧ
��3����֪B����Է�������Ϊ188������ȼ�յIJ�����n��CO2����n��H2O����2��1����B�ķ���ʽΪ ��
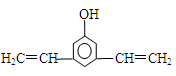
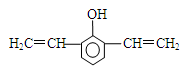
��4��F���������ص㣺������FeCl3��Һ������ɫ��Ӧ���ں˴Ź�����������ʾ���������շ壻�۱����ϵ�һ�ȴ���ֻ�����֣��ܳ������⣬������������״�ṹ��д���������������������ȶ��ṹ��F���ܵĽṹ��ʽ�� ��
��15�֣�
��1���Ȼ���2�֣� CH3CHO+2Cu��OH��2 CH3COOH+Cu2O��+2H2O
CH3COOH+Cu2O��+2H2O
��3�֣�
��2��CH3COOCHBrCH3��2�֣� cd��2�֣�
��3��C12H12O2��2�֣�
��4��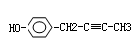

 ����4�֣�
����4�֣�
�������������C��D����ת��ΪE��˵��C��D�е�Cԭ������ͬ������2��Cԭ�ӣ�A������������Һ��Ӧ����A��B��˵��A����������ˮ�������ʹ���C�д���ȩ����˵��C�е��ǻ��Ƿ�������֪�ٵķ�Ӧ��2���ǻ�����ͬһ��Cԭ���ϣ�����A�е�Brԭ���������-C-O�е�C������Aˮ������ɵĴ��Զ���ˮ������CΪ��ȩ����DΪ�����ƣ�E�����ᡣ��1�����ݷ���E�д����Ȼ���C������������ͭ��Ӧ�Ļ�ѧ����ʽΪCH3CHO+2Cu��OH��2 CH3COOH+Cu2O��+2H2O
CH3COOH+Cu2O��+2H2O
��2��A�Ľṹ��ʽΪCH3COOCHBrCH3��A�д�����������ԭ�ӣ����Է���ȡ����Ӧ����ȥ��Ӧ�����Դ�ѡcd��
��3��B����Է���������188����������2��Oԭ�ӣ�����ʣ���C��H����Է���������188-32=156������ȼ�ղ����ж�C:H=1��1�����á����෨������C��H�ĸ�������12������B�ķ���ʽΪC12H12O2��
��4��B������Ӧ���������F����B������������B�ķ���ʽ�ж�B�IJ����Ͷ�Ϊ7��˵������������������ڲ����ͼ�����̼̼������2��̼̼˫����F����10��Cԭ�ӣ�����F���ص��ж�F���б��������ǻ���̼̼������2��̼̼˫���������ϵ�һ�ȴ���ֻ�����֣�˵����������ԭ����2�֣����ǻ���1�֣���������ȡ��������ԭ����2�֡���Fȡ������̼̼��������F����2����λȡ��������F�Ľṹ��ʽΪ
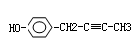 ����Fȡ�����к���2��̼̼˫����2��̼̼˫����������һ����F�Ľṹ��ʽΪ
����Fȡ�����к���2��̼̼˫����2��̼̼˫����������һ����F�Ľṹ��ʽΪ

���㣺�����л��ƶϣ������ŵ��жϼ����ʣ��ṹ��ʽ����ѧ����ʽ���ж�����д������ʽ�ļ��㣬ͬ���칹����ж�����д

 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д� ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д� �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д���15�֣�
���������������ҩ�Ϳ�ϵ�����Ӧ�ù㷺��
��1�����й��ڻ�����I��˵������ȷ���� 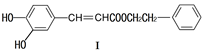
| A����FeCl3��Һ��������ɫ |
| B�����ܷ���������Ӧ��������Ӧ |
| C�������巢��ȡ���ͼӳɷ�Ӧ |
| D��1mol������I�����2molNaOH��Ӧ |

������II�ķ���ʽΪ ��1mol������II���� molH2ǡ�÷�Ӧ���ɱ���������
��3��������II���ɷ����廯����III��IV�ֱ�ͨ����ȥ��Ӧ��á���ֻ��III����Na��Ӧ����H2��III�Ľṹ��ʽΪ ��д1�֣�����IV����II�ķ�Ӧ����Ϊ ��
��4���ۺ���
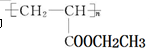 �������Ʊ�Ϳ�ϡ��䵥��Ľṹ��ʽΪ ���������Ʒ�Ӧ�ٵķ���������ϩΪ�л�ԭ�Ϻϳɸõ��壬�漰�ķ�Ӧ����ʽΪ ��
�������Ʊ�Ϳ�ϡ��䵥��Ľṹ��ʽΪ ���������Ʒ�Ӧ�ٵķ���������ϩΪ�л�ԭ�Ϻϳɸõ��壬�漰�ķ�Ӧ����ʽΪ �� ��Ҫ������������⣺
��1��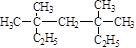 ϵͳ����Ϊ ��
ϵͳ����Ϊ ��
��2��4�D���D1�D��ϩ�ļ���ʽΪ ��
��3��д����ȩ������������ͭ��Ӧ��ѧ����ʽ ��
��4�������������ᷴӦ�������������������д��������������Ľṹ��ʽ
��5�������й�ʵ���˵������ȷ����_____________��
| A������ϩʱ���¶ȼ�Ӧ���뷴Ӧ���Һ�� |
| B��ʵ��������ͱ������۴����·�Ӧ���õ����屽�Ժ�ɫ��ԭ�����屽�ڿ����б����� |
| C������C2H5Cl����Ԫ��ʱ����C2H5Cl��NaOHˮ��Һ��ϼ��ȣ�Ȼ����ϡ�����ữ���ټ���AgNO3��Һ |
| D������������Ӧ���Թ��ð�ˮϴ�ӣ��������ӵ��Թ��þƾ�ϴ�� |






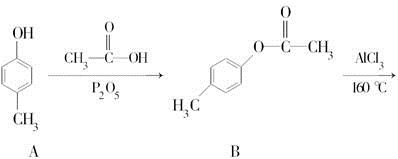



 )��һ����Ҫ�Ļ���ԭ�ϡ���д���Ա�����ȩ���Ҵ�Ϊ��Ҫԭ���Ʊ������������ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ�����£�
)��һ����Ҫ�Ļ���ԭ�ϡ���д���Ա�����ȩ���Ҵ�Ϊ��Ҫԭ���Ʊ������������ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ�����£�