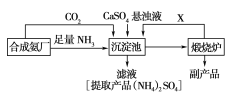
��Ŀ����
����Ŀ����һ��������þ���Ͻ�Ͷ��100 mLһ��Ũ�ȵ������У��Ͻ���ȫ�ܽ⡣��������Һ�еμ�Ũ��Ϊ5 mol/L��NaOH��Һ�����ɵij����������NaOH��Һ�������ϵ��ͼ���������������λ��mL��������������λ��g����
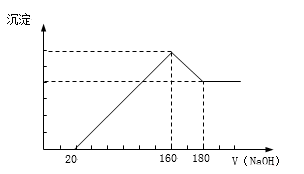
��1������NaOH��Һ0--20mL�����з�Ӧ����ʽΪ��_______________��160--180mL�����з�Ӧ����ʽΪ___________________��
��2���Ͻ���Mg������Ϊ____________g������HCl�����ʵ���Ũ��Ϊ____________mol/L��
���𰸡�
��1��HCl + NaOH�TNaCl+ H2O ��Al��OH��3 + NaOH =NaAlO2 +2 H2O
��2��4.8��8��
��������
�����������1������NaOH��Һ0--20mL��������Һ������������Ȼ����������������������������кͷ�Ӧ��HCl + NaOH�TNaCl+ H2O��160--180mL�����г����������٣����������������������Ʒ����������ܽ⣺Al��OH��3 + NaOH �TNaAlO2 +2 H2O���ʴ�Ϊ��HCl + NaOH�TNaCl+ H2O ��Al��OH��3 + NaOH �TNaAlO2 +2 H2O��
��2��Mg(OH)2��������11.6g��(��Al(OH)3���ڹ�����NaOH����NaAlO2.)
����Mg��Mg(OH)2��2HCl
24 58 2
m(Mg) 11.6 n(HCl)
�������m(Mg)=4.8g
HCl��0.4mol����Ͻ�Ӧ��HCl��ʣ����������0.1mol��NaOH.����HCl��0.1mol��
3HCl��Al��Al(OH)3
3 1 78
n(HCl) 7.8
���n(HCl)=0.3mol��һ������0.4mol +0.1mol +0.3mol =0.8molHCl������c(HCl)=0.8/0.1=8mol/L���ʴ�Ϊ��4.8��8��