��Ŀ����
���غ��������ŷḻ�ĺ�ˮ��Դ����ˮ����Ҫ����Na����K����Ca2����Mg2����Cl����SO42����Br����CO32����HCO3�������ӡ�����������Դ�ͱ��������ǿɳ�����չ����Ҫ��֤�� ��1����ˮ������������Եõ���ˮ�Ȼ�þ����ˮ�Ȼ�þ�ǹ�ҵ��ȡþ��ԭ�ϡ���д����������Ȼ�þ��ȡ����þ�Ļ�ѧ��Ӧ����ʽ ��
��1����ˮ������������Եõ���ˮ�Ȼ�þ����ˮ�Ȼ�þ�ǹ�ҵ��ȡþ��ԭ�ϡ���д����������Ȼ�þ��ȡ����þ�Ļ�ѧ��Ӧ����ʽ ��
��2��ij���������������л��������Cu2����Pb2������ˮ���ŷ�ǰ���ó�������ȥ���������ӣ������������ݣ�����ΪͶ�� ��ѡ�Na2S����NaOH����Ч�����á�
| ���ܵ���� | Cu(OH)2 | CuS | Pb(OH)2 | PbS |
| Ksp | 4��8��10��20 | 6��3��10��36 | 1��2��10��15 | 1��0��10��28 |
��3�������������ҹ�����Դ������ռ�ϴ���أ������ŷų���SO2�����һϵ�л�������̬���⡣���ú�ˮ������һ����Ч�ķ������乤����������ͼ��ʾ��

����Ȼ��ˮ��pH��8���������ӷ���ʽ������Ȼ��ˮ�������Ե�ԭ�� ����дһ������
��ij�о�С��Ϊ̽����ߺ���������SO2����Ч�ʵĴ�ʩ����������Ȼ��ˮ���պ���������ģ��ʵ�飬ʵ������ͼ��ʾ��
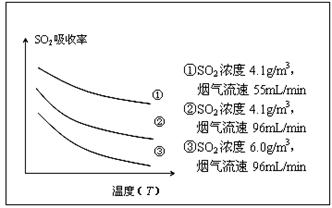
�������ͼʾʵ��������������һ��Ũ�Ⱥ���������SO2������Ч�ʣ����һ�����������飺 ��
����Ȼ��ˮ�����˺��������������H2SO3��HSO3���ȷ��ӻ����ӣ�ʹ���������������Ļ�ѧԭ���� ����дһ����ѧ����ʽ�����ӷ���ʽ����������ġ���ˮ����Ҫ�����������Ȼ��ˮ��֮��Ϻ�����ŷţ��ò�������ҪĿ���� ��
����Դ����������ͷ�չ����Ҫ֧�����о���ѧ��Ӧ�����е������仯����Դ��ȱ�Ľ��������Ҫ���������塣��֪�����Ȼ�ѧ����ʽ
��2H2(g)+O2(g)��2H2O(l)
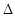 H����570kJ/mol��
H����570kJ/mol����H2(g)+1/2O2(g)��H2O(g)
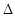 H����242kJ/mol��
H����242kJ/mol����C(s)+1/2O2(g)��CO(g)
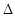 H����110��5kJ/moL��
H����110��5kJ/moL����C(s)+O2(g)��CO2(g)
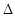 H����393��5kJ/moL��
H����393��5kJ/moL����CO2(g)+2H2O(g)��2CH4(g)+2 O2(g)
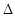 H��+890kJ/moL
H��+890kJ/moL�ش���������
��1��������Ӧ���������ȷ�Ӧ���� ��
��2��H2��ȼ����Ϊ��H�� ��
��3����˹�����������Ϳ�ѧ�о����к���Ҫ�����塣��Щ��Ӧ�ķ�Ӧ����Ȼ��ֱ�Ӳⶨ������ͨ����ӵķ�����á���֪C(s) + H2O(g)��H2(g)+ CO(g)
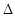 H��akJ/moL����a�� ���÷�Ӧ����
H��akJ/moL����a�� ���÷�Ӧ����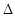 S 0(ѡ���������������������)����֪������
S 0(ѡ���������������������)����֪������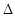 G��
G��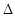 H��T
H��T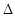 S����
S����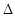 G��0ʱ���Է����С���÷�Ӧ��ʲô�����¿��Է�����__________________��
G��0ʱ���Է����С���÷�Ӧ��ʲô�����¿��Է�����__________________����4��CO��������ȼ�ϵ��Ϊ����ԭ������װ����ͼ��ʾ���õ���е����Ϊ�����ƣ������ƣ�����O2-�����ڹ������NASICON�������ƶ�������˵��������� ��
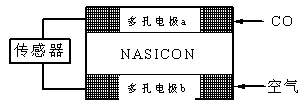
A�������ĵ缫��ӦʽΪ��CO+O2���D2e-��CO2
B������ʱ�缫b��������O2���ɵ缫a����缫b
C������ʱ�����ɵ缫aͨ������������缫b
D����������ͨ���ĵ���Խ��β����CO�ĺ���Խ��
����15�֣���1�� MgCl2�����ڣ� Mg��Cl2��1�� ��2�� Na2S 1��
Mg��Cl2��1�� ��2�� Na2S 1��
��3����CO32����H2O HCO3����OH���� HCO3����H2O
HCO3����OH���� HCO3����H2O H2CO3��OH�� 1��
H2CO3��OH�� 1��
�ڽ��ͺ����������¶ȣ����С�������������٣��� 1��
��2H2SO3��O2��4H����2SO42������2H2SO4����2HSO3����O2��2H����2SO42����2��
�к͡�ϡ�;�����������ˮ�����ɵ��ᣨH+����������Ⱦ��1��
��1�� �� 1�� ��2����285kJ/mol 1��
��3����131.5 2�� �� 1�� ���� 1�� ��4��B 2��
�������������I����1��þ�ǻ��õĽ�����ұ��ʱ��Ҫͨ��������ڵ��Ȼ�þ���У���Ӧ�Ļ�ѧ����ʽΪMgCl2�����ڣ� Mg��Cl2����
Mg��Cl2����
��2�����ݱ������ݿ�֪���Ȼ�ͭ�������ܶȻ������ֱ�ԶС��������ͭ�������������ܶȻ����������Գ��������ѡ�����ơ�
��3�������ں�ˮ�к���CO32����HCO3�������ӣ����߾�ˮ��ʹ��ˮ�Լ��ԣ���Ӧ�����ӷ���ʽΪCO32����H2O HCO3����OH���� HCO3����H2O
HCO3����OH���� HCO3����H2O H2CO3��OH����
H2CO3��OH����
�ڸ���ͼ���֪���¶�Խ�ߣ�SO2��������ԽС�����¶���ͬʱ����������ԽС��SO2��������Խ�����Ժ�������Ӧ���ǽ��ͺ����������¶ȣ����С�������������٣���
������H2SO3��HSO3���ȷ��ӻ����Ӿ����л�ԭ�ԣ��ױ�����ΪSO42������Ӧ�����ӷ���ʽΪ2H2SO3��O2��4H����2SO42������2H2SO4����2HSO3����O2��2H����2SO42�������ڷ�Ӧ����Һ��������ǿ������������ġ���ˮ����Ҫ�����������Ȼ��ˮ��֮��Ϻ�����ŷţ��ò�������ҪĿ�����к͡�ϡ�;�����������ˮ�����ɵ��ᣨH+����������Ⱦ��
��1�����Ȼ�ѧ����ʽ�С�H��0��ʾ��Ӧ�Ƿ��ȷ�Ӧ����H��0��ʾ��Ӧ�����ȷ�Ӧ������������Ӧ���������ȷ�Ӧ���Ǣݣ�������Ƿ��ȷ�Ӧ��
��2��ȼ��������һ�������£�1mol��ȼ����ȫȼ�������ȶ���������ʱ���ų�������������H2��ȼ����Ϊ��H����570kJ/mol��2����285kJ/mol��
��3����֪��H2(g)+1/2O2(g)��H2O(g) ��H����242kJ/mol����C(s)+1/2O2(g)��CO(g) ��H����110��5kJ/moL������ݸ�˹���ɿ�֪�ۣ��ڼ��õ�C(s) + H2O(g)��H2(g)+ CO(g)�����Ը÷�Ӧ�ķ�Ӧ�ȡ�H����110��5kJ/moL��242kJ/mol����131.5 kJ/mol����a����131.5�����ݷ���ʽ��֪���÷�Ӧ���������ģ����Ը÷�Ӧ���ء�S��0�����ݡ�G����H��T����S��֪������G��0ʱ���Է����С����ڸ÷�Ӧ�ġ�S��0����H��0����÷�ӦӦ���ڸ��������¿��Է����С�
��4��ԭ����и���ʧȥ���ӣ�����������Ӧ�������õ����ӣ�������ԭ��Ӧ�������ڸ�ȼ�ϵ����CO�ٸ���ͨ�룬����������ͨ�롣A��CO�ڸ���ͨ�룬���ĵ缫��ӦʽΪ��CO+O2���D2e-��CO2��A��ȷ��B������ʱ�缫b������������ԭ��������������ƶ�������O2���ɵ缫b����缫a��B����ȷ��C��ԭ����е��ӴӸ����������������Թ���ʱ�����ɵ缫aͨ������������缫b��C��ȷ��D����������ͨ���ĵ���Խ�����ĵ�COԽ�࣬����β����CO�ĺ���Խ�ߣ�D��ȷ����ѡB��
���㣺�������ұ�����ܶȻ�����Ӧ�á�����ˮ�⡢��Ӧ�ȡ���Ӧ�Է����Լ�ȼ�ϵ�ص��й��ж�

�״���һ����Ҫ�Ļ���ԭ�ϡ��״���ˮ�����������ɻ�������Դ�����й㷺��Ӧ��ǰ������������ʵ�飬�����Ϊ1 L���ܱ������У�����1mol CH3OH��1molH2O��һ�������·�����Ӧ��CH3OH (g)+ H2O (g) CO2(g) +3 H2 (g)�����CO2��CH3OH(g)��Ũ����ʱ��仯���±���ʾ��
CO2(g) +3 H2 (g)�����CO2��CH3OH(g)��Ũ����ʱ��仯���±���ʾ��
| ʱ�� ���� | 0 min | 10 min | 30 min | 60 min | 70 min |
| CO2(mol/L) | 0 | 0.2 | 0.6 | 0.8 | 0.8 |
| CH3OH(mol/L) | 1.0 | 0.8 | 0.4 | 0.2 | 0.2 |
����֪��CH3OH (g)+
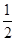 O2 (g)
O2 (g) CO2(g) + 2H2 (g) ?H1= ��192.9kJ/mol
CO2(g) + 2H2 (g) ?H1= ��192.9kJ/mol H2(g)+
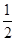 O2 (g)
O2 (g) H2 O(g) ?H2= ��120.9kJ/mol
H2 O(g) ?H2= ��120.9kJ/mol ��״���ˮ������������Ӧ���ʱ��H3=_____ ��
��10~30 min�ڣ�������ƽ����Ӧ����v(H2)��___________mol/(L��min)��
�۸÷�Ӧ��ƽ�ⳣ������ʽΪK=__________________��
�����д�ʩ����ʹƽ��ʱn(CH3OH)��n(CO2)��С����(˫ѡ)___________��
A��������� B�����ݳ���He(g)��ʹ��ϵѹǿ����
C����H2(g)����ϵ�з��� D���ٳ���1molH2O
��2���״��ڴ��������¿���ֱ�������ɼ��ᡣ
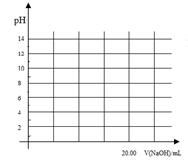
���ڳ����£���0.1000 mol/L NaOH��Һ�ζ�20. 00 mL 0.1000 mol/L ������Һ�����У������Һ��pH=7ʱ�������ĵ�V(NaOH)___(�������������) 20. 00 mL��
���������ζ������У��������ỻ�����ᣬ����ͼ�е���Ӧλ�û�����Ӧ�ĵζ����ߡ�(1����ҺԼ0.04mL)
���������ѳ�Ϊ��ǰ��δ����һ��ȫ�����ش���⡣Ϊ���Ŀǰȼ��ʹ�ù����еĻ�����Ⱦ���⣬��������ԴΣ�����е�ר���������̫���ܴ�ʹȼ��ѭ��ʹ�õĹ��룬��ͼ��ʾ��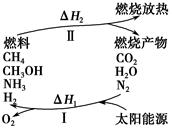
���̢�������·�Ӧ��ʾ��
��2CO2 2CO��O2����2H2O===2H2��O2����2N2��6H2O
2CO��O2����2H2O===2H2��O2����2N2��6H2O 4NH3��3O2����2CO2��4H2O
4NH3��3O2����2CO2��4H2O 2CH3OH��3O2����2CO��4H2O
2CH3OH��3O2����2CO��4H2O ________��3O2
________��3O2
��ش��������⣺
(1)���̢������ת����ʽΪ________��ת��Ϊ________�ܡ�
(2)����ɵڢݸ���Ӧ�Ļ�ѧ����ʽ��____________________��
(3)����ת�������У���H1�ͦ�H2�Ĺ�ϵ��________��
(4)����1 mol��ѧ��������������±���
| ���ۼ� | H��N | H��O | N��N | O===O |
| ����1 mol��ѧ����������/(kJ��mol��1) | 393 | 460 | 941 | 499 |
�����£�N2��H2O��Ӧ����NH3���Ȼ�ѧ����ʽΪ_________��
��ѧ��һֱ�����ڡ��˹��̵����ķ����о���
(1)�ϳɰ���ԭ��Ϊ��N2(g)+3H2(g) 2NH3(g)��H="-92.4" kJ/mol���÷�Ӧ�������仯��ͼ��ʾ��
2NH3(g)��H="-92.4" kJ/mol���÷�Ӧ�������仯��ͼ��ʾ��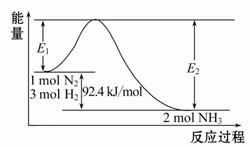
���ڷ�Ӧ��ϵ�м����������Ӧ��������E2�ı仯�� (���������С�����䡱)��
�ڽ�0.3 mol N2��0.5 mol H2�������������ܱ������У���һ�������´ﵽƽ�⣬�������������ѹǿ��Ϊԭ����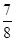 ����ʱH2��ת����Ϊ ������߸�������H2��ת���ʣ����д�ʩ���е��� (��ѡ����ĸ)��
����ʱH2��ת����Ϊ ������߸�������H2��ת���ʣ����д�ʩ���е��� (��ѡ����ĸ)��
| A���������а�ԭ�����ٳ���ԭ���� |
| B�����������ٳ���һ����H2 |
| C���ı䷴Ӧ�Ĵ��� |
| D��Һ�������������� |
2N2(g)+6H2O(l)
 4NH3(g)+3O2(g)
4NH3(g)+3O2(g)��H="+1" 530 kJ/mol
��֪��H2O(l)
 H2O(g) ��H="+44.0" kJ/mol
H2O(g) ��H="+44.0" kJ/mol��2N2(g)+6H2O(g)
 4NH3(g)+3O2(g) ��H= kJ/mol���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK �����������������䣬����ѹǿ��Kֵ (���������С�����䡱)��
4NH3(g)+3O2(g) ��H= kJ/mol���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK �����������������䣬����ѹǿ��Kֵ (���������С�����䡱)�� ͭ���ʼ��仯�����ڹ�ҵ�����Ϳ���������Ҫ���á�
��1����֪��2Cu2O(s) + O2(g) = 4CuO(s)��H����292kJ��mol��1
2C(s)+O2(g)=2CO(g) ��H����221kJ��mol��1
��д��������̿�ۻ�ԭCuO��s���Ʊ�Cu2O��s�����Ȼ�ѧ����ʽ�� ��
��2�������Ȼ�ͭ����(CuCl2��2H2O�����Ȼ���������)��ȡ������CuCl2��2H2O���Ƚ����Ƴ�ˮ��Һ������ͼ��������ᴿ: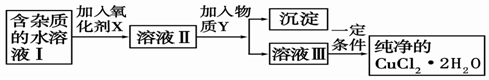
��֪Cu2+��Fe3+��Fe2+���������↑ʼ�����ͳ�����ȫʱ��pH���±�
| �������� | Fe3+ | Fe2+ | Cu2+ |
| �������↑ʼ����ʱ��pH | 1.9 | 7.0 | 4.7 |
| ����������ȫ����ʱ��pH | 3.2 | 9.0 | 6.7 |
��ش���������:
������������NaClO��H2O2��KMnO4��X�����ֺã�Ϊʲô������������������������������������������������������
�÷�Ӧ�����ӷ���ʽΪ ��
����ҺII�г�Cu2+�⣬���������������������ӣ���μ�������� ��
������Y����Ϊ���е�
a��CuO b��Cu(OH)2 c��CuCO3 d��Cu2(OH)2CO3 e��CaO f��NaOH
��������Һ���м���̼��ƣ�������������������������������������������
�����Ź㷺����;�������ڻ��ʡ����ᡢ�ϳ���ά�ȹ�ҵ������
��1���������¡��˹��̵������о��������ڳ��¡���ѹ�����������£�N2�ڴ�������������Fe2O3��TiO2��������ˮ������Ӧ�����ɰ�����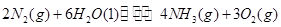
�÷�Ӧ�ڹ̶�������ܱ������н��У��й�˵����ȷ����_____________���������ĸ����
A����Ӧ����ƽ��״̬ʱ��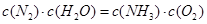 |
B����Ӧ�ﵽƽ���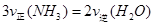 |
| C����ϵ����ѹǿ���䣬˵����Ӧ�Ѵ�ƽ�� |
| D�����������ܶȱ��ֲ��䣬˵����Ӧ�Ѵ�ƽ�� |
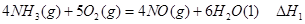 ��
��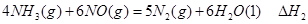 ��
��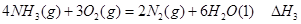 ��
����д������������Ӧ��
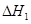 ��
��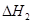 ��
��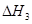 ����֮���ϵ�ı���ʽ��
����֮���ϵ�ı���ʽ��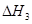 ��_________��
��_________����3����ҵ���������Ҫ��Ӧ�ǣ�
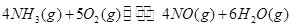
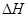 ��
��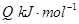
�������¶ȣ���Ӧ��Kֵ��С����Q______���>������<��������0��
������Ӧ��ʼ�����ʵ�����ͬ�����й�ϵͼ�������________������ţ���


�����ݻ��̶����ܱ������з���������Ӧ�������ڲ������ʵ�Ũ�����±���
| ʱ��/Ũ�� |  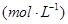 | 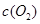 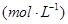 | 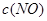 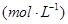 | 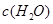 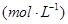 |
| ��ʼ | 4.0 | 5.5 | 0 | 0 |
| ��2min | 3.2 | a | 0.8 | 1.2 |
| ��4min | 2.0 | 3.0 | 2.0 | 3.0 |
| ��6min | 2.0 | 3.0 | 2.0 | 3.0 |
��Ӧ�ڵ�2 min����4 minʱ��O2��ƽ����Ӧ����Ϊ________��
��Ӧ�ڵ�2 minʱ�ı����������ı������������______________________________��
�������£���Ӧ��ƽ�ⳣ��K��________��
����β���ﺬ�е�NO������������ȼ��ȼ�յĸ�����������������Ӧ���£�
N2(g)��O2(g)  2NO(g) ��H����֪�÷�Ӧ�� T ��ʱ��ƽ�ⳣ��K��9.0��
2NO(g) ��H����֪�÷�Ӧ�� T ��ʱ��ƽ�ⳣ��K��9.0��
��ش�
��1����֪��N2(g)+2O2(g)  2NO2(g) ��H1 2NO2(g)
2NO2(g) ��H1 2NO2(g)  O2+2NO(g) ��H2 ��H= ���ú���H1����H2�ı���ʽ��ʾ����
O2+2NO(g) ��H2 ��H= ���ú���H1����H2�ı���ʽ��ʾ����
��2��ij�¶��£���2 L���ܱ������г���N2��O2��1 mol��5���Ӻ�O2�����ʵ���Ϊ0.5 mol����NO�ķ�Ӧ���� ��
��3���ٶ��÷�Ӧ���ں��������½��У��������жϸ÷�Ӧ�Ѵﵽƽ�����________��
| A������1 mol N2ͬʱ����1 mol O2 |
| B����������ܶȲ��� |
| C���������ƽ����Է����������� |
| D��2v��(N2)��v��(NO) |
 2NO(g)�ġ�K-T������c(NO)-t��ͼ����ͼA������֪�÷�ӦΪ ��Ӧ������ȡ����ȡ�������ͼB��֪����a��Ӧ��������ȣ�b�ı������������ ��
2NO(g)�ġ�K-T������c(NO)-t��ͼ����ͼA������֪�÷�ӦΪ ��Ӧ������ȡ����ȡ�������ͼB��֪����a��Ӧ��������ȣ�b�ı������������ ��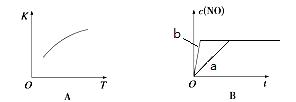
��5��T ��ʱ��ijʱ�̲��������N2��O2��NO��Ũ�ȷֱ�Ϊ0.20 mol��L��1��0.20mol��L��1��0.50mol��L��1����ʱ��ӦN2(g)��O2(g)
 2NO(g)________________(����ڻ�ѧƽ��״̬������������Ӧ������С������淴Ӧ������С�)��ƽ��ʱ��N2�ڻ�����������ٷ���Ϊ���٣����ڴ����д�����������̣��������2λ��Ч���֣�
2NO(g)________________(����ڻ�ѧƽ��״̬������������Ӧ������С������淴Ӧ������С�)��ƽ��ʱ��N2�ڻ�����������ٷ���Ϊ���٣����ڴ����д�����������̣��������2λ��Ч���֣�  CH3OH��g������H1����90 kJ��mol��1
CH3OH��g������H1����90 kJ��mol��1
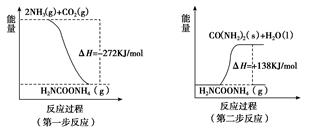
 CH3OH(g)����H����90.1 kJ��mol��1 ��һ��ѹǿ�£��ݻ�ΪV L�������г���a mol CO��2a mol H2���ڴ��������·�Ӧ���ɼ״���ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
CH3OH(g)����H����90.1 kJ��mol��1 ��һ��ѹǿ�£��ݻ�ΪV L�������г���a mol CO��2a mol H2���ڴ��������·�Ӧ���ɼ״���ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
