��Ŀ����
��12�֣����ڹ�����ռ����Ҫ��λ��
��1���ϳɰ���ҵ�У��ϳ�����ÿ����2 mol NH3���ų�92.2 kJ������
�ٹ�ҵ�ϳɰ����Ȼ�ѧ����ʽ��_______��
������ʼʱ�������ڷ���2 mol N2��6 mol H2����ƽ���ų�������ΪQ����Q���>������<����=����_______184.4 kJ��
����֪��

1 mol N-H���������յ�����Լ����_______kJ��
��2����ҵ�������ص�ԭ������NH3��CO2Ϊԭ�Ϻϳ�����[CO(NH2)2]����Ӧ�Ļ�ѧ����ʽΪ2NH3 (g)+ CO2 (g) CO(NH2)2 (l) + H2O (l)���÷�Ӧ��ƽ�ⳣ�����¶ȹ�ϵ���£�
CO(NH2)2 (l) + H2O (l)���÷�Ӧ��ƽ�ⳣ�����¶ȹ�ϵ���£�
���ʱ䦤H���>������<����=����_______0��
����һ���¶Ⱥ�ѹǿ�£���ԭ�����е�NH3��CO2�����ʵ���֮�ȣ���̼�ȣ�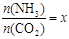 ����ͼ�ǰ�̼�ȣ�x����CO2ƽ��ת���ʣ������Ĺ�ϵ��������x����������ԭ����_______��
����ͼ�ǰ�̼�ȣ�x����CO2ƽ��ת���ʣ������Ĺ�ϵ��������x����������ԭ����_______��
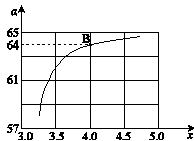
����ͼ�е�B�㴦��NH3��ƽ��ת����Ϊ_______��
��1���ϳɰ���ҵ�У��ϳ�����ÿ����2 mol NH3���ų�92.2 kJ������
�ٹ�ҵ�ϳɰ����Ȼ�ѧ����ʽ��_______��
������ʼʱ�������ڷ���2 mol N2��6 mol H2����ƽ���ų�������ΪQ����Q���>������<����=����_______184.4 kJ��
����֪��

1 mol N-H���������յ�����Լ����_______kJ��
��2����ҵ�������ص�ԭ������NH3��CO2Ϊԭ�Ϻϳ�����[CO(NH2)2]����Ӧ�Ļ�ѧ����ʽΪ2NH3 (g)+ CO2 (g)
 CO(NH2)2 (l) + H2O (l)���÷�Ӧ��ƽ�ⳣ�����¶ȹ�ϵ���£�
CO(NH2)2 (l) + H2O (l)���÷�Ӧ��ƽ�ⳣ�����¶ȹ�ϵ���£�| T / �� | 165 | 175 | 185 | 195 |
| K | 111.9 | 74.1 | 50.6 | 34.8 |
����һ���¶Ⱥ�ѹǿ�£���ԭ�����е�NH3��CO2�����ʵ���֮�ȣ���̼�ȣ�
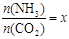 ����ͼ�ǰ�̼�ȣ�x����CO2ƽ��ת���ʣ������Ĺ�ϵ��������x����������ԭ����_______��
����ͼ�ǰ�̼�ȣ�x����CO2ƽ��ת���ʣ������Ĺ�ϵ��������x����������ԭ����_______��
����ͼ�е�B�㴦��NH3��ƽ��ת����Ϊ_______��
��1����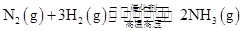
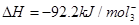
�� < ��391
��2���� < ��c(NH3)����ƽ�������ƶ� ��32%
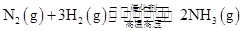
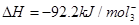
�� < ��391
��2���� < ��c(NH3)����ƽ�������ƶ� ��32%
�����������1���ϳɰ���ҵ��ÿ����2 mol NH3���ų�92.2 kJ���������ݶ�����ϵ���Եõ��ٹ�ҵ�ϳɰ����Ȼ�ѧ����ʽ�Ǣ�
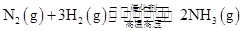
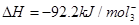 �����ڸ÷�Ӧ�ǿ��淴Ӧ����2 mol N2��6 mol H2��ȫ��Ӧ�����Ͽ��Էų�184.4 kJ����������ʵ���еõ���ֻ�ܱ�184.4 kJ�����Ԣ�����ʼʱ�������ڷ���2 mol N2��6 mol H2����ƽ���ų�������ΪQ<184.4 kJ��
�����ڸ÷�Ӧ�ǿ��淴Ӧ����2 mol N2��6 mol H2��ȫ��Ӧ�����Ͽ��Էų�184.4 kJ����������ʵ���еõ���ֻ�ܱ�184.4 kJ�����Ԣ�����ʼʱ�������ڷ���2 mol N2��6 mol H2����ƽ���ų�������ΪQ<184.4 kJ������֪��

�����ɷ�Ӧ�ȵļ��㹫ʽ�ɵ�6 mol N-H���ܡ���1 mol N-N����+3 mol H-H���ܣ�=92.2kj
����1 mol N-H���������յ�����Լ����391kJ��
��2���ɸ÷�Ӧ��ƽ�ⳣ�����¶ȹ�ϵ
| T / �� | 165 | 175 | 185 | 195 |
| K | 111.9 | 74.1 | 50.6 | 34.8 |
���Կ����¶�Խ�ߣ�ƽ�������ȷ����ƶ���KԽС��ƽ�����淴Ӧ�����ƶ������淴Ӧ����������ȷ�����������Ӧ����Ϊ���ȷ�Ӧ�����Ԣ��ʱ䦤H<0��
�� 2NH3 (g)+ CO2 (g)
 CO(NH2)2 (l) + H2O (l)����NH3��Ũ�ȣ�ƽ���������Ӧ�����ƶ�������CO2ƽ��ת���ʻ�����
CO(NH2)2 (l) + H2O (l)����NH3��Ũ�ȣ�ƽ���������Ӧ�����ƶ�������CO2ƽ��ת���ʻ����� 2NH3 (g)+ CO2 (g)
 CO(NH2)2 (l) + H2O (l)
CO(NH2)2 (l) + H2O (l)��ʼ�����ʵ����� 4x x 0 0
�仯�����ʵ����� 2y y y y
ƽ�������ʵ����� 4x-2y x-y y y
CO2ƽ��ת����a=y/x=64%
���Ԣ���ͼ�е�B�㴦��NH3��ƽ��ת����=2y/4x=32%
���������û�ѧ�����㷴Ӧ�ȣ���������ʽ���л�ѧƽ��ļ��㣬���������û�ѧƽ�ⳣ�����¶ȵı仯�������淴Ӧ�������������������ʽ��������һ�б�ʾ�������ʿ�ʼ��Ӧ�������ڶ��б�ʾ�������ʷ�Ӧ�����������б�ʾ�������ʷ�Ӧ�������

��ϰ��ϵ�д�
�����Ŀ
 H2(g)+
H2(g)+ 
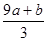 mol
mol 2SO3(g) Ϊ���ȷ�Ӧ����SO2������һ������SO3������
2SO3(g) Ϊ���ȷ�Ӧ����SO2������һ������SO3������