��Ŀ����
17��ij��Һ�п��ܺ����������ӣ�Na+��SO${\;}_{4}^{2-}$��Ba2+��NO${\;}_{3}^{-}$��OH-��NH${\;}_{4}^{+}$�е�ij�������ӣ�Ϊȷ������Һ����ɣ�ijͬѧ��������ʵ�飨��������1��ȡ����������Һ�������м�����������Һ��������ɫ����������ϡ����������ܽ⣻
��2����ȡ��������Һ�������м���������NaOH��Һ�����ȣ������д̼�����ζ�����壮�������й�˵���в���ȷ���ǣ�������
| A�� | ����Һ��һ������NH${\;}_{4}^{+}$ | |
| B�� | ����Һ��һ��������������� | |
| C�� | ����Һ��һ������SO${\;}_{4}^{2-}$ | |
| D�� | ��������ʵ�鲻��ȷ��Na+�Ƿ���� |
���� ��1��ȡ����������Һ�������м�����������Һ��������ɫ����������ϡ����������ܽ⣬��֪���ɵij���ΪBaSO4����Һ��һ����Ba2+����Ba2+��SO42-��ͬһ��Һ�ﲻ���棬�ʿ϶�����SO42-��
��2����ȡ��������Һ�������м���������NaOH��Һ�����ȣ������д̼�����ζ�����壬������ΪNH3����֪��Һ����NH4+����NH4+��OH-��ͬһ��Һ�ﲻ�ܴ������棬�ʿ϶�����OH-��
��� �⣺��1��ȡ����������Һ�������м�����������Һ��������ɫ����������ϡ����������ܽ⣬��֪���ɵij���ΪBaSO4����Һ��һ����Ba2+����Ba2+��SO42-��ͬһ��Һ�ﲻ���棬�ʿ϶�����SO42-����2����ȡ��������Һ�������м���������NaOH��Һ�����ȣ������д̼�����ζ�����壬������ΪNH3����֪��Һ����NH4+����NH4+��OH-��ͬһ��Һ�ﲻ�ܴ������棬�ʿ϶�����OH-����ϣ�1���ͣ�2����֪����Һ��϶����С����϶������С�������Һ�ǵ������ǣ���֪���Ķ��������ӣ��ʱ������һ�������ӣ���SO${\;}_{4}^{2-}$��NO${\;}_{3}^{-}$��OH-ֻ����NO3-һ�����ڣ��������������ȷ��Na+�Ƿ���ڣ���ѡC��
���� ���⿼�����ӵļ�����ƶϣ���Ŀ�Ѷ��еȣ�ע��������ӷ�Ӧ��ʵ�������Լ����ӵļ����ʵ������������ؼ����ܺ�����Һ�ǵ����Եģ��й�NO3-���ж����ѵ㣮

 ʱ�����������ҵԭ���ܳ�����ϵ�д�
ʱ�����������ҵԭ���ܳ�����ϵ�д� ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�| A�� | ˮ�����մɡ���������Ҫ�ɷֶ��ǹ����� | |
| B�� | �Ͻ���۵�ͨ���ȽM�ֽ����ͣ�Ӳ�ȱ���Ϸֽ����� | |
| C�� | ���г��ּ�������Ҫ�ǵ绯ѧ��ʴ���� | |
| D�� | ������ϩ������ʳƷ��װ�� |
| A�� | ��ѧ�����ڴ�������Һ�е����̪��Һ�����ȣ�������ˮ������������ɫ���˵��������ˮ�������ȵ� | |
| B�� | ��ѧ����������茶�������ˮ����ˮ���½���˵�������ˮ�������ȵ� | |
| C�� | ��ѧ����ȡһ��Ũ�ȵ�FeCl3��Һ���ڱ�ˮ��һ��ʱ�䣬������Һ�ػ�ɫ��dz��˵��FeCl3��ˮ�������ȵ� | |
| D�� | ��ѧ����ʵ��ⶨͬŨ�ȵ��ȵĴ���Һ����Ĵ���ҺpH�ߣ�˵��̼����ˮ�������ȵ� |
| A�� | NH3��O2��ʼʱ���ʵ���֮�ȵ���4��5����ƽ��������ʵ�ת����һ����� | |
| B�� | ƽ��ʱ4v����O2��=5v����NO�� | |
| C�� | ƽ���ѹ���������ƽ��Ħ��������С | |
| D�� | �ﵽƽ��ʱ�ų�����Ϊ905.9 kJ������ʼʱͶ��4mol NH3��5mol O2 |
��1�����о�������230�桫270��ʱ�ϳ���Ϊ������Ϊ̽Ѱ�ϳ�������ʵ���ʼ��ɱȣ��ֱ���230�桢250���270��ʱ����ʵ�飬ʵ������ͼ��230���ʵ��������Ӧ��������X������ĸ���� �����COת���ʵĽǶȵ��ۺϷ��������¶��¹�ҵ�������˲��õĺϳ������n��H2����n��CO���ı�ֵ��Χ��B������ĸ����
A��1��1.5��������B��2.5��3C��3.5��4.5

��2���Ƽ״�����Ҫ����������ͨ�����з�Ӧ��ȡ��H2O��g��+CO��g��?H2��g��+CO2��g������H��0��ij�¶��¸÷�Ӧ��ƽ�ⳣ��K=1���ش��������⣺
�ٸ��¶��£�����ʼʱc��CO��=2mol•L-1��c��H2O��=3mol•L-1����Ӧ����һ��ʱ����CO��Ũ��Ϊ1mol•L-1�����ʱ�÷�Ӧv��������v���棩�����������������=������
���������¶ȣ��÷�Ӧ��Kֵ���������������С�����䡱����
��3���״���һ�ֻ���ԭ�ϣ���ҵ�Ϻϳɼ״��ķ�Ӧ��CO��g��+2H2��g��?CH3OH��g����H=-90.8kJ•mol-1��
�����¶ȡ��ݻ���ͬ��3���ܱ������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��¡����ݣ���÷�Ӧ�ﵽƽ��ʱ���й��������£�
| ���� | �� | �� | �� |
| ��Ӧ��Ͷ���� | 1molCO��2molH2 | 1mol CH3OH | 2molCO��4molH2 |
| CH3OH��Ũ�ȣ�mol/L�� | c1 | c2 | c3 |
| ��Ӧ�������仯 | �ų�Q1 kJ | ����Q2 kJ | �ų�Q3 kJ |
�ڱ仯��������ֵQ�У�Q1 ��Q2�ĺ���90.8���������ֵ����
��4��Ŀǰ���Լ״�Ϊԭ�ϵ�ȼ�ϵ���Ѿ�Ӧ���ڹ�ҵ��������ͼ�Ǽ״�ȼ�ϵ��Ӧ�õ�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��2CH3OH+3O2+4KOH�T2K2CO3+6H2O��
 ��
���������ͼ��д���пհף�
�ҳ���A�缫�ĵ缫��ӦʽΪAg++e-=Ag���׳���ͨ��CH3OH�缫�ĵ缫��ӦʽΪCH3OH-6e-+8OH-=CO32-+6H2O��
| A�� | Ũ�������ǿ�����ԣ������������ۻ�������A1��Fe���������桢����Ũ���� | |
| B�� | �������а뵼�����ʣ��������������ά | |
| C�� | ����������Ư���ԣ������ڼӹ�ʳƷʹʳƷ���� | |
| D�� | Fe3+���������ԣ������ھ�ˮ |
| A�� | ����ʱ�������ˮ�����˿̶��ߣ�����ý�ͷ�ι��������ಿ�� | |
| B�� | ����ʱ��Ӧ���¶ȼ�ˮ��������������ƿ֧�ܿڴ� | |
| C�� | ��Һʱ���²�Һ��ӷ�Һ©���¿��������ϲ�Һ��Ӧ�ӷ�Һ©���Ͽڵ��� | |
| D�� | ����NaOH ʱ��NaOH ����С�ձ��з���������ƽ���̣������������ |
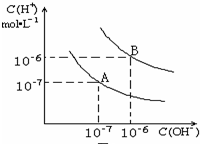 ˮ�ĵ���ƽ��������ͼ��ʾ��
ˮ�ĵ���ƽ��������ͼ��ʾ��