��Ŀ����
��֪��������



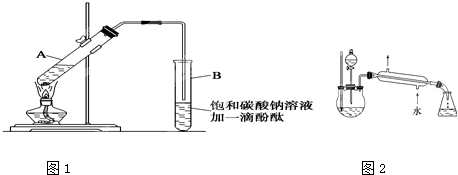
ʵ������������������Ҫװ������ͼI��ʾ����Ҫ����Ϊ��
����30mL�Ĵ��Թ��а������1��4��4�ı�������Ũ���ᡢ�Ҵ�������Ļ��Һ��
�ڰ���ͼI����װ�ã�ʹ����������������ͨ��15mL�Թ���ʢ����Na2CO3��Һ(����1�η�̪��Һ)�Ϸ�2mm~3mm����
��С������Թ��еĻ��Һ��
�ܴ�С�Թ����ռ�Լ4mL����ʱֹͣ���ȣ�����С�Թܲ�������Ȼ���ô���ֲ㣻
�ݷ��������������������
��ͬѧ�ǻش��������⣺
��1��������У�������һ�����Ļ��Һ�IJ�����_________��
��2��д���÷�Ӧ�Ļ�ѧ����ʽ_______________��ŨH2SO4��������______________��
��3��������У���С������Թ��еĻ��Һ����ԭ�������_________________��
��4����������۲쵽��������______________��ԭ����____________________��
��5��������У��������������ѡ�õ�������_________������Ӧ��_________�ڵ�������Ϊ__________________��
��6��Ϊ������������IJ��ʣ��ס�����λͬѧ�ֱ����������ͼ�ס��ҵ�װ��(��ͬѧ����Ӧ�����ȴ�����ñ���Na2CO3��Һ��ȡ��ƿ�в���)������Ϊ����װ�ú�����Ϊʲô����___________________________��
����30mL�Ĵ��Թ��а������1��4��4�ı�������Ũ���ᡢ�Ҵ�������Ļ��Һ��
�ڰ���ͼI����װ�ã�ʹ����������������ͨ��15mL�Թ���ʢ����Na2CO3��Һ(����1�η�̪��Һ)�Ϸ�2mm~3mm����
��С������Թ��еĻ��Һ��
�ܴ�С�Թ����ռ�Լ4mL����ʱֹͣ���ȣ�����С�Թܲ�������Ȼ���ô���ֲ㣻
�ݷ��������������������
��ͬѧ�ǻش��������⣺
��1��������У�������һ�����Ļ��Һ�IJ�����_________��
��2��д���÷�Ӧ�Ļ�ѧ����ʽ_______________��ŨH2SO4��������______________��
��3��������У���С������Թ��еĻ��Һ����ԭ�������_________________��
��4����������۲쵽��������______________��ԭ����____________________��
��5��������У��������������ѡ�õ�������_________������Ӧ��_________�ڵ�������Ϊ__________________��
��6��Ϊ������������IJ��ʣ��ס�����λͬѧ�ֱ����������ͼ�ס��ҵ�װ��(��ͬѧ����Ӧ�����ȴ�����ñ���Na2CO3��Һ��ȡ��ƿ�в���)������Ϊ����װ�ú�����Ϊʲô����___________________________��
��1���ȼ�������Ҵ���4mL���ٻ�������1mLŨH2SO4���ӱ���
��2��CH3COOH+CH3CH2OH CH3COOCH2CH3+H2O����������ˮ��
CH3COOCH2CH3+H2O����������ˮ��
��3�����ᡢ�Ҵ������������е�ӽ��ҽϵͣ������ȣ���Ӧ�����������ʧ
��4����dz��ɫNa2CO3��Һ�ϲ���Լ4cm�����ɫҺ�壬��Na2CO3��Һ���ɫ��dz�������ݣ��ϲ�Һ��䱡���ϲ����Ͳ���Ϊ���ɵ���������������ˮ�����ܶȱ�ˮС��ͬʱ��Ϊ�ӷ�������������̼���Ʒ�Ӧ���ų�CO2���壬���������ݳ���
��5����Һ©�����ϣ�����������ˮ�ܶ�С
��6���ң���Ӧ������������
��2��CH3COOH+CH3CH2OH
 CH3COOCH2CH3+H2O����������ˮ��
CH3COOCH2CH3+H2O����������ˮ����3�����ᡢ�Ҵ������������е�ӽ��ҽϵͣ������ȣ���Ӧ�����������ʧ
��4����dz��ɫNa2CO3��Һ�ϲ���Լ4cm�����ɫҺ�壬��Na2CO3��Һ���ɫ��dz�������ݣ��ϲ�Һ��䱡���ϲ����Ͳ���Ϊ���ɵ���������������ˮ�����ܶȱ�ˮС��ͬʱ��Ϊ�ӷ�������������̼���Ʒ�Ӧ���ų�CO2���壬���������ݳ���
��5����Һ©�����ϣ�����������ˮ�ܶ�С
��6���ң���Ӧ������������

��ϰ��ϵ�д�
 �ľ�ͼ���ʱ�ȷ�ϵ�д�
�ľ�ͼ���ʱ�ȷ�ϵ�д�
�����Ŀ
��֪�������ݣ�
2Fe��s��+O2��g��=2FeO��s����H=-544kJ?mol-1
4Al��s��+3O2��g��=2Al2O3��s����H=-3350kJ?mol-1
��2Al��s��+3FeO��s��=Al2O3��s��+3Fe��s���ġ�H�ǣ�������
2Fe��s��+O2��g��=2FeO��s����H=-544kJ?mol-1
4Al��s��+3O2��g��=2Al2O3��s����H=-3350kJ?mol-1
��2Al��s��+3FeO��s��=Al2O3��s��+3Fe��s���ġ�H�ǣ�������
| A��-859 kJ?mol-1 | B��+859 kJ?mol-1 | C��-1403 kJ?mol-1 | D��-2491 kJ?mol-1 |
 ij��ѧ��Ӧ�У��跴Ӧ���������ΪE1���������������ΪE2��
ij��ѧ��Ӧ�У��跴Ӧ���������ΪE1���������������ΪE2��