��Ŀ����
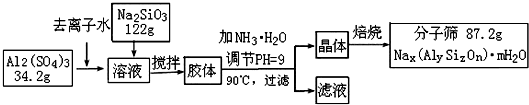
(12��)����ɸ���������ʵķ����ᴿ��ij���ͺŵķ���ɸ�Ĺ�ҵ�������̿ɼ�ʾ���£�
![]()
��1���ڼ�NH3��H2O����pH�Ĺ����У���pH���Ʋ�������Al(OH)3���ɡ���֪�����£�Al(OH)3��Һ��pH=3����Ksp=1��10-36��������c(Al3+)=���� �� ��
��2������������������Һ����Ҫ�ɷ�Ϊ���� �� ��д��ѧʽ����
��3������������������Ԫ�غ�Ԫ�ؾ�û����ģ���ԭ�ӵ�������Ϊ10������ͨ������ȷ���÷���ɸ�Ļ�ѧʽ��д��������̣���
��4������ɸ�Ŀ�ֱ��Ϊ4Å��Ϊ4A�ͷ���ɸ����Na+��Ca2+ȡ��ʱ���Ƶ�5A�ͷ���ɸ����Na+��K+ȡ��ʱ���Ƶ�3A�ͷ���ɸ��Ҫ��Ч���������飨����ֱ��Ϊ4.65Å�����춡�飨����ֱ��Ϊ5.6Å��Ӧ��ѡ�������� �͵ķ���ɸ��
����:

 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д� ��˼άС�ھ�100����ҵ��ϵ�д�
��˼άС�ھ�100����ҵ��ϵ�д� ��ʦָ��һ��ͨϵ�д�
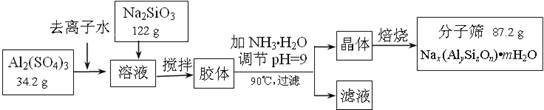
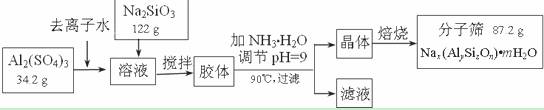
��ʦָ��һ��ͨϵ�д�(12��)����ɸ���������ʵķ����ᴿ��ij���ͺŵķ���ɸ�Ĺ�ҵ�������̿ɼ�ʾ���£�
|
��1���ڼ�NH3��H2O����pH�Ĺ����У���pH���Ʋ�������Al(OH)3���ɡ���֪�����£�Al(OH)3��Һ��pH=3����Ksp=1��10-36��������c(Al3+)=���� �� ��
��2������������������Һ����Ҫ�ɷ�Ϊ���� �� ��д��ѧʽ����
��3������������������Ԫ�غ�Ԫ�ؾ�û����ģ���ԭ�ӵ�������Ϊ10������ͨ������ȷ���÷���ɸ�Ļ�ѧʽ��д��������̣���
��4������ɸ�Ŀ�ֱ��Ϊ4Å��Ϊ4A�ͷ���ɸ����Na+��Ca2+ȡ��ʱ���Ƶ�5A�ͷ���ɸ����Na+��K+ȡ��ʱ���Ƶ�3A�ͷ���ɸ��Ҫ��Ч���������飨����ֱ��Ϊ4.65Å�����춡�飨����ֱ��Ϊ5.6Å��Ӧ��ѡ������ �� �͵ķ���ɸ��