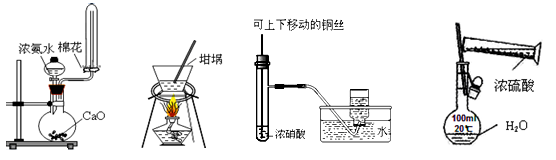
��Ŀ����
��12�֣���Na2CO3��10H2O���壬����0.2 mol/L��Na2CO3��Һ480 mL��
(1)ʵ�����õ��IJ�����������Ͳ�����������ձ�����ȱ��_____________��____________��
(2)Ӧ��������ƽ��ȡNa2CO3��10H2O�ľ��������Ϊ_________________g��
(3)������Һʱ�����¼������������ܽ⡡��ҡ�ȡ���ϴ�Ӳ�ת��ϴ��Һ������ȴ���ݳ�������ת����Һ �߶���
��ȷ�IJ���˳����__________________(�����)��
(4)�������в�����������Һ��Ũ�ȸ���ʲôӰ�죬�����ա�
��̼����ʧȥ�˲��ֽᾧˮ�� ���á���������ij��������������塡
��̼���ƾ��岻�������л����Ȼ��� �ܳ���̼���ƾ���ʱ�����������⡡
������ƿδ�������ʹ��
��������������ҺŨ��ƫ�ߵ���_________����Ӱ�����________��(�����)
(1)ʵ�����õ��IJ�����������Ͳ�����������ձ�����ȱ��_____________��____________��
(2)Ӧ��������ƽ��ȡNa2CO3��10H2O�ľ��������Ϊ_________________g��
(3)������Һʱ�����¼������������ܽ⡡��ҡ�ȡ���ϴ�Ӳ�ת��ϴ��Һ������ȴ���ݳ�������ת����Һ �߶���
��ȷ�IJ���˳����__________________(�����)��
(4)�������в�����������Һ��Ũ�ȸ���ʲôӰ�죬�����ա�
��̼����ʧȥ�˲��ֽᾧˮ�� ���á���������ij��������������塡
��̼���ƾ��岻�������л����Ȼ��� �ܳ���̼���ƾ���ʱ�����������⡡
������ƿδ�������ʹ��
��������������ҺŨ��ƫ�ߵ���_________����Ӱ�����________��(�����)
��12�֣�ÿ��2�֣���1�� 500 mL����ƿ����ͷ�ι� (2)28.6 g
(3)�ݢ٢ܢޢۢߢ� (4)�٢ܣ�2�֣���һ����1�֣���+ѡ���÷֣�������
(3)�ݢ٢ܢޢۢߢ� (4)�٢ܣ�2�֣���һ����1�֣���+ѡ���÷֣�������
����һ�����ʵ���Ũ����Һ�����Ƽ��������ȡ�
��1����������ƿ�Ĺ��û��480ml�ģ�����Ӧ������500ml������Ҫ500ml����ƿ�Լ�����ʱ�Ľ�ͷ�ιܡ�
��2��500ml0.2mol/L��̼������Һ�У�̼���Ƶ����ʵ�����0.1mol��������ҪNa2CO3��10H2O�����������0.1mol��286g/mol��28.6g��
��3������һ�����ʵ���Ũ����Һ������ԭ����Ҫ���֪����ȷ�IJ���˳���Ǣݢ٢ܢޢۢߢڡ�
��4������c��n/V��֪��̼����ʧȥ�˲��ֽᾧˮ�����������ӣ�Ũ��ƫ�ߣ��á���������ij��������������壬���ʼ��٣�Ũ��ƫС��̼���ƾ��岻�������л����Ȼ��ƣ����ʼ��٣�Ũ��ƫС������̼���ƾ���ʱ�����������⣬���������ӣ�Ũ��ƫ�ߣ�����ƿ���������Ӱ��ʵ��������ѡAB��
��1����������ƿ�Ĺ��û��480ml�ģ�����Ӧ������500ml������Ҫ500ml����ƿ�Լ�����ʱ�Ľ�ͷ�ιܡ�
��2��500ml0.2mol/L��̼������Һ�У�̼���Ƶ����ʵ�����0.1mol��������ҪNa2CO3��10H2O�����������0.1mol��286g/mol��28.6g��
��3������һ�����ʵ���Ũ����Һ������ԭ����Ҫ���֪����ȷ�IJ���˳���Ǣݢ٢ܢޢۢߢڡ�
��4������c��n/V��֪��̼����ʧȥ�˲��ֽᾧˮ�����������ӣ�Ũ��ƫ�ߣ��á���������ij��������������壬���ʼ��٣�Ũ��ƫС��̼���ƾ��岻�������л����Ȼ��ƣ����ʼ��٣�Ũ��ƫС������̼���ƾ���ʱ�����������⣬���������ӣ�Ũ��ƫ�ߣ�����ƿ���������Ӱ��ʵ��������ѡAB��

��ϰ��ϵ�д�
�����Ŀ