��Ŀ����
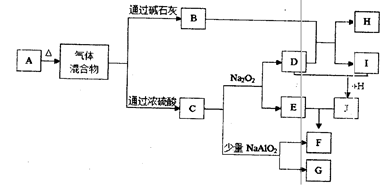
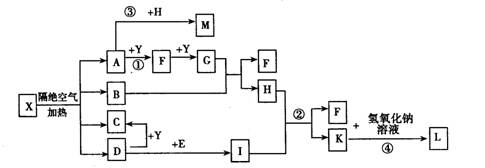
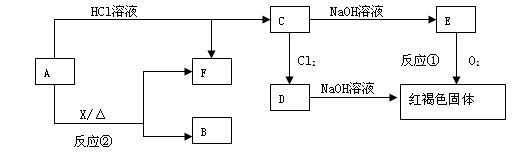
��֪XΪһ���Σ�A��C��D��FΪ��ɫ���壬B���³�ѹ��Ϊ��ɫ��ζ��Һ�壬I��YΪ��ѧ��ѧ�����ĵ��ʣ�����YΪ���壬IΪ�Ϻ�ɫ���壬EΪ��ɫ���������LΪ��ɫ������������Щ��Ӧ�����������������ﱻ��ȥ��
�ش��������⣺
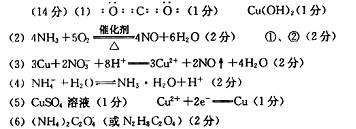
��1��д��C�ĵ���ʽ ��L�Ļ�ѧʽ ��
��2��д����Ӧ�ٵĻ�ѧ��Ӧ����ʽ_ ___����Ӧ��һ��������������ԭ��Ӧ���� ��
��3��д����Ӧ�ڵ����ӷ�Ӧ����ʽ__ __��
��4��M��ˮ��Һ�����ԣ���ԭ��Ϊ�������ӷ���ʽ��ʾ��__ __��
��5�����õ����ᴿI���ʣ��ڸõ�ⷴӦ�е������Һ��__ __��д�������ĵ缫��Ӧʽ___ _��
��6����֪1 mol X�ڸ������������·ֽ�����ĸ���������ʵ���֮��ΪA:B:C:D=2��1��1��1���Ʋ�A�Ļ�ѧʽΪ___ _��

�ش��������⣺
��1��д��C�ĵ���ʽ ��L�Ļ�ѧʽ ��
��2��д����Ӧ�ٵĻ�ѧ��Ӧ����ʽ_ ___����Ӧ��һ��������������ԭ��Ӧ���� ��
��3��д����Ӧ�ڵ����ӷ�Ӧ����ʽ__ __��
��4��M��ˮ��Һ�����ԣ���ԭ��Ϊ�������ӷ���ʽ��ʾ��__ __��
��5�����õ����ᴿI���ʣ��ڸõ�ⷴӦ�е������Һ��__ __��д�������ĵ缫��Ӧʽ___ _��
��6����֪1 mol X�ڸ������������·ֽ�����ĸ���������ʵ���֮��ΪA:B:C:D=2��1��1��1���Ʋ�A�Ļ�ѧʽΪ___ _��
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 �õ�H��Һ�������������������ͬ����Ԫ���γɵļ��������л�ԭ����ǿ����������Ԫ�أ��������ʼ�ת����ϵ����ͼ�������ַ�Ӧ�����������ȥ��
�õ�H��Һ�������������������ͬ����Ԫ���γɵļ��������л�ԭ����ǿ����������Ԫ�أ��������ʼ�ת����ϵ����ͼ�������ַ�Ӧ�����������ȥ��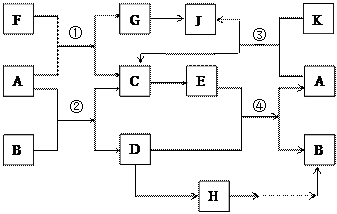

 2CA3��g��
2CA3��g�� .0 L��
.0 L��