题目内容
写出下列难溶电解质的溶度积表达式。
Mg(OH)2__________________AgBr__________________
Ca3(PO4)2__________________Ag2S__________________
Mg(OH)2__________________AgBr__________________
Ca3(PO4)2__________________Ag2S__________________
Ksp[Mg(OH)2]=[c(Mg2+)]·[c(OH-)]2
Ksp[AgBr]=[c(Ag+)]·[c(Br-)]
Ksp[Ca3(PO4)2]=[c(Ca2+)]3·[c(PO43-)]2
Ksp[Ag2S]=[c(Ag+)]2·[c(S2-)]
Ksp[AgBr]=[c(Ag+)]·[c(Br-)]
Ksp[Ca3(PO4)2]=[c(Ca2+)]3·[c(PO43-)]2
Ksp[Ag2S]=[c(Ag+)]2·[c(S2-)]

练习册系列答案
 期末100分闯关海淀考王系列答案
期末100分闯关海淀考王系列答案 小学能力测试卷系列答案
小学能力测试卷系列答案
相关题目
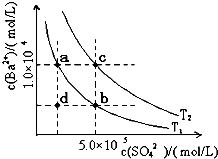

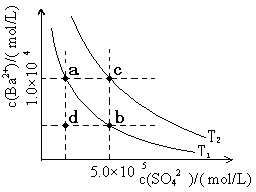
 1.7mol/L)处理。若使BaSO4中的SO42-全部转化到溶液中,需要反复处理 次。[提示: BaSO4(s)+ CO32- (aq)
1.7mol/L)处理。若使BaSO4中的SO42-全部转化到溶液中,需要反复处理 次。[提示: BaSO4(s)+ CO32- (aq) BaCO3(s)+ SO42- (aq) ]
BaCO3(s)+ SO42- (aq) ]