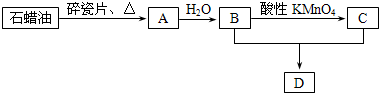
��Ŀ����
(17��)��A��B��C��D��E 5��Ԫ�أ����ǵĺ˵�������������Ҷ�С��20������C��E�ǽ���Ԫ�أ�A��E��ͬһ�壬����ԭ�ӵ����������Ų�Ϊns1��B��DҲ��ͬһ�壬����ԭ��������p�ܼ���������s�ܼ���������������Cԭ��������ϵ���������Dԭ��������ϵ�������һ�롣��ش��������⣺
��1��A��________��B��________��E��_________��
��2��д��CԪ�ػ�̬ԭ�ӵĵ����Ų�ʽ_________________________��
��3��B ��D����Ԫ�ص��⻯���У��е����� ��д��ѧʽ�ţ�����ԭ����
��4��Ԫ��B��D�ĵ縺�ԵĴ�С��ϵ��___________��C��E�ĵ�һ�����ܵĴ�С��ϵ��___________�����>������<����=������Ԫ�ط��ű�ʾ��
��5��Eλ�����ڱ��ĵ� ���ڣ��� �壻��ԭ�ӽṹʾ��ͼΪ ��
��6��A��B�γɵ�A2B2�����ﺬ ��������ԡ��Ǽ��ԡ������� ���ӡ�������ԡ��Ǽ��ԡ��������ʽΪ:______________________________��
��
����:

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�