��Ŀ����
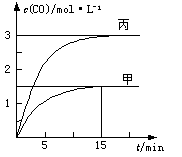
һ�������´��ڷ�Ӧ��C(s)��H2O(g) CO(g)��H2(g)��H>0����ס��ҡ����������������м���һ����C��H2O�����������¶ȡ���Ӧ�����ʼ�����±�����Ӧ������CO�����ʵ���Ũ����ʱ��仯��ͼ��ʾ��
CO(g)��H2(g)��H>0����ס��ҡ����������������м���һ����C��H2O�����������¶ȡ���Ӧ�����ʼ�����±�����Ӧ������CO�����ʵ���Ũ����ʱ��仯��ͼ��ʾ��
����˵����ȷ����
A���������У���Ӧ��ǰ15 min��ƽ������v(H2)="0.1" mol��L��1��min��1
B�������������V<0.5 L
C�����¶�ΪT1 ��ʱ����Ӧ��ƽ�ⳣ��K=2.25
D���������У���ƽ��ʱn(H2O)="0.4" mol����T1< T2
 CO(g)��H2(g)��H>0����ס��ҡ����������������м���һ����C��H2O�����������¶ȡ���Ӧ�����ʼ�����±�����Ӧ������CO�����ʵ���Ũ����ʱ��仯��ͼ��ʾ��
CO(g)��H2(g)��H>0����ס��ҡ����������������м���һ����C��H2O�����������¶ȡ���Ӧ�����ʼ�����±�����Ӧ������CO�����ʵ���Ũ����ʱ��仯��ͼ��ʾ��
| ���� | �� | �� | �� |
| �ݻ� | 0.5 L | 0.5 L | V |
| �¶� | T1 �� | T2 �� | T1 �� |
| ��ʼ�� | 2 molC 1 molH2O | 1 molCO 1 molH2 | 4 molC 2 molH2O |
A���������У���Ӧ��ǰ15 min��ƽ������v(H2)="0.1" mol��L��1��min��1
B�������������V<0.5 L
C�����¶�ΪT1 ��ʱ����Ӧ��ƽ�ⳣ��K=2.25
D���������У���ƽ��ʱn(H2O)="0.4" mol����T1< T2
AB
���������A�����ͼ�м�������CO��ƽ��Ũ�ȿɵ���ǰ15 min��ƽ������v(H2)��
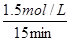 ��0.1 mol��L��1��min��1����ȷ��B������������V��0.5 L�����ݵ�Чƽ��ԭ��CO��ƽ��Ũ��ӦС��3.0mol/L��ֻ�������С����ʹCO��ƽ��Ũ�ȴﵽ3.0mol/L����ȷ��C��ݼ�������ƽ��Ũ�ȼ���ƽ�ⳣ��K��
��0.1 mol��L��1��min��1����ȷ��B������������V��0.5 L�����ݵ�Чƽ��ԭ��CO��ƽ��Ũ��ӦС��3.0mol/L��ֻ�������С����ʹCO��ƽ��Ũ�ȴﵽ3.0mol/L����ȷ��C��ݼ�������ƽ��Ũ�ȼ���ƽ�ⳣ��K��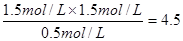 ������D�����ڹ������ʲ�Ӱ��ƽ�⣬��ס�������������ͬ���ܴﵽȫ��ƽ�⣬��n(H2O)=0.5mol��0.4mol���ٽ������Ӧ���ȣ�˵�����������¶ȵͣ�T1��T2��������
������D�����ڹ������ʲ�Ӱ��ƽ�⣬��ס�������������ͬ���ܴﵽȫ��ƽ�⣬��n(H2O)=0.5mol��0.4mol���ٽ������Ӧ���ȣ�˵�����������¶ȵͣ�T1��T2��������
��ϰ��ϵ�д�
�����Ŀ
 ʵ���ò�ͬ�¶��µ�ƽ�����������±���
ʵ���ò�ͬ�¶��µ�ƽ�����������±���
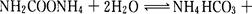
 ���о�С��ֱ������ݲ�ͬ��ʼŨ�ȵİ����������Һ�ⶨˮ�ⷴӦ���ʣ��õ�C(NH2C00-)��ʱ��ı仯������ͼ��ʾ��
���о�С��ֱ������ݲ�ͬ��ʼŨ�ȵİ����������Һ�ⶨˮ�ⷴӦ���ʣ��õ�C(NH2C00-)��ʱ��ı仯������ͼ��ʾ��

 HCOOH(l) + CH3OH(l)����Ӧ���ȣ����ʱ��ֵ��С�����³�ѹ�£�ˮ�ⷴӦ���ʺ�ƽ�ⳣ������С��
HCOOH(l) + CH3OH(l)����Ӧ���ȣ����ʱ��ֵ��С�����³�ѹ�£�ˮ�ⷴӦ���ʺ�ƽ�ⳣ������С��
 N2(g)+3H2(g)����673K��30MPa�£�n(NH3)��n(N2)��ʱ��仯�Ĺ�ϵ��ͼ��ʾ������������ȷ����
N2(g)+3H2(g)����673K��30MPa�£�n(NH3)��n(N2)��ʱ��仯�Ĺ�ϵ��ͼ��ʾ������������ȷ���� 
 M(g)��N(g)������ʵ���������±���
M(g)��N(g)������ʵ���������±���
 3Z(g) ��H��0��W��X������Ӧ���ʵ���M��X������Ӧ����
3Z(g) ��H��0��W��X������Ӧ���ʵ���M��X������Ӧ����