��Ŀ����
����Ŀ��A��B��C������Ԫ�ص�ԭ�ӣ���������С��18��Aԭ����Bԭ�ӵ���������������6��Aԭ����Cԭ�ӵĺ�����Ӳ�����Ϊ3�� Cԭ�ӵ���������A��4���ش��������⣺
��1��A��B��C��Ԫ�����Ʒֱ�Ϊ________��________��________��
��2��A�����ӽṹʾ��ͼΪ________��
��3��Ԫ��A��һ��������Ϊ32�ĺ���,��Ԫ��B��һ�ֺ��ع�����ΪAB42-��1mol AB42-������Ϊ104g,��Ԫ��B�ĸú����е�������Ϊ_______��
���𰸡� �� �� þ ![]() 10
10
��������A��B��C������Ԫ�ص�ԭ�ӣ���������С��18��Aԭ����Bԭ�ӵ���������������6�����ǵ���AԪ�أ�Aԭ����Cԭ�ӵĺ�����Ӳ�����Ϊ3������A��S��B��O��Cԭ�ӵ���������A��4����C��Mg����1���������Ϸ�����֪A��B��C��Ԫ�����Ʒֱ�Ϊ��������þ����2��������ӽṹʾ��ͼΪΪ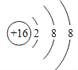 ����3��Ԫ��A��һ��������Ϊ32�ĺ�������Ԫ��B��һ�ֺ��ع�����ΪSO42����1molSO42��������Ϊ104g����Ԫ��B����������(104��32)/4��18����˸ú����е�������Ϊ18��8��10��
����3��Ԫ��A��һ��������Ϊ32�ĺ�������Ԫ��B��һ�ֺ��ع�����ΪSO42����1molSO42��������Ϊ104g����Ԫ��B����������(104��32)/4��18����˸ú����е�������Ϊ18��8��10��

��ϰ��ϵ�д�
�����Ŀ


