��Ŀ����
6��ij�л���X��C12H13O6Br�������к��ж��ֹ����ţ���ṹ��ʽΪ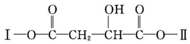 �����Т�Ϊδ֪���ֵĽṹ����
�����Т�Ϊδ֪���ֵĽṹ����Ϊ�Ʋ�X�ķ��ӽṹ��������ͼ��ת����

��֪��E��ˮ��Һ�е���FeCl3��Һ������ɫ��Ӧ��M��C2H2O4����ʹ��īˮ��ɫ��G��M������NaHCO3��Һ��Ӧ��
��ش�
��1��M�Ľṹ��ʽΪHOOC-COOH��G�������������ŵ��������Ȼ����ǻ���
��2��E���Է����ķ�Ӧ�У�ѡ����ţ��٢ۢܣ�
�ټӳɷ�Ӧ������ȥ��Ӧ����������Ӧ����ȡ����Ӧ
��3����Bת����D�Ļ�ѧ����ʽ��HOCH2-CH2OH+O2$��_{��}^{����}$OHC-CHO+2H2O
��4��G��һ�������·�����Ӧ���ɷ������ΪC4H4O4���л�����л����ʹ������Ȼ�̼��Һ��ɫ����д��G�����˷�Ӧ�Ļ�ѧ����ʽHOOC-CH2-CH��OH��-COOH$\stackrel{һ������}{��}$HOOC-CH=CH-COOH+H2O��
���� ��E��ˮ��Һ�е���FeCl3��Һ����ɫ��Ӧ��E����-OH����˵��X�к��б����������л���X�ķ���ʽC12H13O6Br�������Ͷ�Ϊ$\frac{2��12+2-13-1}{2}$=6�������֪���ֵĽṹ�����ж�X�����г��˱���������̼��˫���⣬û�����������ͼ���
��ת����ϵ��B������������M��M�ķ���ʽΪC2H2O4����֪BΪOHCH2CH2OH��DΪOHCCHO��MΪHOOCCOOH��
����л���X�ķ���ʽ����֪���ֽṹ��֪��E�Ľṹ��ʽΪ ��AΪ
��AΪ ��G��M������NaHCO3��Һ��Ӧ��G����NaHCO3��Һ��Ӧ�������Ȼ�������л���X�ķ���ʽ�����еĽṹ
��G��M������NaHCO3��Һ��Ӧ��G����NaHCO3��Һ��Ӧ�������Ȼ�������л���X�ķ���ʽ�����еĽṹ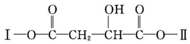 ��֪GΪ
��֪GΪ ��NΪ
��NΪ ����X�Ľṹ��ʽΪ
����X�Ľṹ��ʽΪ ���Դ������
���Դ������
��� �⣺��1��������������֪��MΪ�Ҷ��ᣬ�ṹ��ʽΪHOOC-COOH��GΪ �����й���������Ϊ�Ȼ����ǻ���
�����й���������Ϊ�Ȼ����ǻ���
�ʴ�Ϊ��HOOC-COOH���Ȼ����ǻ���
��2��E�Ľṹ��ʽΪ ���������ɷ����ӳɷ�Ӧ����-OH�ɷ���������ȡ����Ӧ�����ܷ�����ȥ��Ӧ����E���Է����ķ�Ӧ�Т٢ۢܣ�
���������ɷ����ӳɷ�Ӧ����-OH�ɷ���������ȡ����Ӧ�����ܷ�����ȥ��Ӧ����E���Է����ķ�Ӧ�Т٢ۢܣ�
�ʴ�Ϊ���٢ۢܣ�
��3��Bת����DΪ�Ҷ������������������Ҷ�ȩ����Ӧ����ʽΪHOCH2-CH2OH+O2$��_{��}^{����}$OHC-CHO+2H2O��
�ʴ�Ϊ��HOCH2-CH2OH+O2$��_{��}^{����}$OHC-CHO+2H2O��
��4��GΪ ����һ�������·�����Ӧ���ɷ������ΪC4H4O4���л�����л����ʹ������Ȼ�̼��Һ��ɫ�����в����ͼ�����Ϊ�ǻ�������ȥ��Ӧ��������HOOC-CH=CH-COOH����Ӧ����ʽΪHOOC-CH2-CH��OH��-COOH$\stackrel{һ������}{��}$HOOC-CH=CH-COOH+H2O��
����һ�������·�����Ӧ���ɷ������ΪC4H4O4���л�����л����ʹ������Ȼ�̼��Һ��ɫ�����в����ͼ�����Ϊ�ǻ�������ȥ��Ӧ��������HOOC-CH=CH-COOH����Ӧ����ʽΪHOOC-CH2-CH��OH��-COOH$\stackrel{һ������}{��}$HOOC-CH=CH-COOH+H2O��
�ʴ�Ϊ��HOOC-CH2-CH��OH��-COOH$\stackrel{һ������}{��}$HOOC-CH=CH-COOH+H2O��
���� ���⿼���л�����ƶϣ�Ϊ��Ƶ���㣬����ϰ���е���Ϣ�������ŵı仯���л���ӦΪ���Ĺؼ������ط������ƶ������Ŀ��飬ע��X�Ľṹ�ƶ�Ϊ�����ѵ㣬��Ŀ�ѶȲ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ��2a=bʱ����Һ�����ɵij���������� | |
| B�� | ��a=2bʱ�����������ӷ�ӦΪ2NH4++SO42-+Ba2++2OH-�TBaSO4��+2NH3•H2O | |
| C�� | ��2b��3aʱ�����������ӷ�ӦΪ3SO42-+2Al3++3Ba2++6OH-�T3BaSO4��+2Al��OH��3�� | |
| D�� | ��2a��b��2.5aʱ����Һ�е�n��AlO2-��Ϊ0.02��b-2a�� mol |
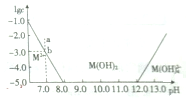
| A�� | M��OH��2���������������� | |
| B�� | ��������M2+�����pH��8��12֮�� | |
| C�� | �����¶ȣ�����ʵ�ִ�b���ƶ���a�� | |
| D�� | ����ʱ��M��OH��2��s�����ܶȻ�����Ϊ1��10-17 |
| ���� | HCOOH | HCN | H2S |
| ����ƽ�ⳣ����25�棩 | Ka=1.8��10-4 | Ka=4.9��10-10 | Ka1=1.3��10-7 Ka2=7.1��10-15 |
| A�� | NaHS��Һ�м�������KOH��c��Na+���Tc��H2S��+c��HS-��+2C��S2-�� | |
| B�� | HCOO-��CN-��HS-����Һ�в����Դ������� | |
| C�� | ���������Ũ�ȵ�HCOONa��NaCN����Һ������������Ŀǰ�ߴ��ں��� | |
| D�� | ǡ���к͵��������pH��HCOOH��HCN����NaOH����ǰ�ߴ��ں��� |
| A�� | 4.6 g NO2��N2O4�Ļ��������������ԭ����Ϊ0.1NA | |
| B�� | ���³�ѹ��1.6 g�����������õ��Ӷ���Ϊ0.1NA | |
| C�� | ��״���£�6.72 L CO2������NO2��Ӧת�Ƶ�����Ϊ0.6NA | |
| D�� | 50 mL 98%Ũ���ᣨ�ܶ�Ϊ1.84 g•cm-3��������ͭ���ȣ�ת�Ƶĵ�����Ϊ 0.92NA |

| A�� | ����60 mL 0.50 mol/L�����50 mL 0.55 mol/L NaOH��Һ���з�Ӧ��������к�����ֵ��ԭ����ͬ | |
| B�� | ��50 mL 0.50 mol/L�����50 mL 0.55 mol/L NaOH����ʵ����� 50 mL 0.50 mol/L�����50 mL 0.50 mol/L NaOH��õ���ֵȷ | |
| C�� | �����ʱ����Ͳ��NaOH��ҺӦ��������С�ձ��У������ò��������� | |
| D�� | װ���еĴ�С�ձ�֮����������ĭ���ϵ������DZ��¸��ȡ�����������ʧ |
