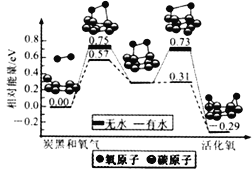
��Ŀ����
����Ŀ����1�����ݻ�Ϊ2 L���ܱ������У���CO2��H2�ϳɼ״����������������������£������¶ȶԷ�Ӧ��Ӱ�죬ʵ��������ͼ��ʾ(ע��T1��T2������300��)��
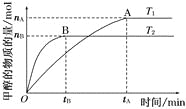
����˵����ȷ����_______(�����)��
���¶�ΪT1ʱ���ӷ�Ӧ��ʼ��ƽ�⣬���ɼ״���ƽ������Ϊv(CH3OH)��![]() mol��L��1��min��1
mol��L��1��min��1
�ڸ÷�Ӧ��T1ʱ��ƽ�ⳣ����T2ʱ��С
�۸÷�ӦΪ���ȷ�Ӧ
�ܴ���A��ķ�Ӧ��ϵ��T1�䵽T2���ﵽƽ��ʱ![]() ����
����
(2)��T1�¶�ʱ����1 mol CO2��3 mol H2����һ�ܱպ��������У���ַ�Ӧ�ﵽƽ�����CO2��ת����Ϊ�����������ڵ�ѹǿ����ʼѹǿ֮��Ϊ_______��
���𰸡��ۢ� 1��![]()
��������
(1)����ͼ���е����ݿ�֪���¶�T1ʱ���ɼ״������ʣ�nA/2tAmol��L��1��min��1���ʢٴ���ͼ��֪���¶ȴ�T1���ߵ�T2ʱ���״������ʵ������ͣ�˵��ƽ�������ƶ����ʴ�ʱƽ�ⳣ����С���ʢڴ�����Ϊ�����¶ȣ�ƽ�������ƶ������淴Ӧ�����ȷ�Ӧ����������Ӧ�Ƿ��ȷ�Ӧ���ʢ���ȷ����Ϊ�¶ȴ�T1���ߵ�T2ʱ��ƽ�������ƶ��������������ʵ������״������ʵ�����С����n(H2)/n(CH3OH)���ʢ���ȷ��
(2)д������ʽCO2��3H2![]() CH3OH��H2O����ƽ��ʱCO2��H2��CH3OH��H2O�仯�����ʵ����ֱ����� mol��3�� mol���� mol���� mol����ƽ��ʱ���ǵ����ʵ����ֱ���(1����) mol��(3��3��) mol���� mol���� mol����ƽ��ʱ����������ʵ�����(4��2��) mol�����Դ�ʱ��ѹǿ����ʼѹǿ֮��Ϊ(4��2��)/4��(2����)/2��
CH3OH��H2O����ƽ��ʱCO2��H2��CH3OH��H2O�仯�����ʵ����ֱ����� mol��3�� mol���� mol���� mol����ƽ��ʱ���ǵ����ʵ����ֱ���(1����) mol��(3��3��) mol���� mol���� mol����ƽ��ʱ����������ʵ�����(4��2��) mol�����Դ�ʱ��ѹǿ����ʼѹǿ֮��Ϊ(4��2��)/4��(2����)/2��