��Ŀ����
����Ŀ����(![]() )�뱽Ȳ(
)�뱽Ȳ(![]() )��Ӧ���ɻ�����X(����Գ�ͼ��)����ͼ��ʾ��
)��Ӧ���ɻ�����X(����Գ�ͼ��)����ͼ��ʾ��
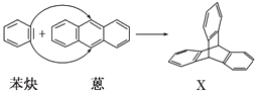
(1)����X������__��
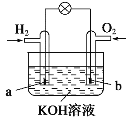
a����������b����������c��������
(2)��Ȳ�ķ���ʽΪ__����Ȳ�����е�������__��
a��������ˮ b���ܷ���������Ӧ c���ܷ����ӳɷ�Ӧ d�����³�ѹ��Ϊ����
(3)�������ڱ���ͬϵ�����__(����ĸ����)��
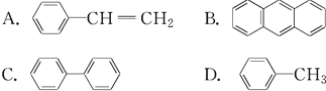
(4)�ܷ����ӳɷ�Ӧ��Ҳ�ܷ���ȡ����Ӧ��ͬʱ��ʹ��ˮ��Ӧ��ɫ��Ҳ��ʹ���Ը��������Һ��ɫ����__��
A.![]() B.C6H14 C.
B.C6H14 C.![]() D.
D.![]()
���𰸡�ac C6H4 ad D D
��������
��1��������������״�ṹ��
��2���ɽṹ��ʽ��֪����ʽ����̼̼˫����
��3������ͬϵ���к�1������������Ϊ����������
��4���ܷ����ӳɡ�ȡ������ѡ���֪����̼̼˫����
(1)������������״�ṹ��������X�����ڻ���������������
�ʴ�Ϊ��ac��
(2)�ɽṹ��ʽ��֪����ʽΪC6H4����̼̼�������ܷ����ӳɡ�������Ӧ����������ˮ��������ΪҺ�壬
�ʴ�Ϊ��C6H4��ad��
(3)����ͬϵ���к�1������������Ϊ����������ֻ��D���ϣ�
�ʴ�ѡD��
(4)�ܷ����ӳɡ�������ȡ������ѡ���֪����������̼̼˫����ֻ��ѡ��D���ϣ�
�ʴ�ѡD��