��Ŀ����
(9��)��֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������A ������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ���塣B��C��ͬһ���壬D��E��ͬһ���ڣ���֪E�����ڱ���1��18���еĵ�7��Ԫ�ء�D��ԭ��������EС5��D��B���γ����ӻ������侧���ṹ����ͼ�� 
 ��1��A��Ԫ�ط����� ��E��Ԫ�����ڱ��е�λ���� ������+2�����ӵĵ����Ų�ʽΪ ��
��1��A��Ԫ�ط����� ��E��Ԫ�����ڱ��е�λ���� ������+2�����ӵĵ����Ų�ʽΪ ��  ��2��B���⻯��ľ��������� ���壬B���⻯����C���⻯����ȣ����Ӽ��Խϴ���� ��д��ѧʽ����
��2��B���⻯��ľ��������� ���壬B���⻯����C���⻯����ȣ����Ӽ��Խϴ���� ��д��ѧʽ���� ��3����ͼ�п��Կ�����D��B�γɵ����ӻ�����ĵ���ʽΪ �������ӻ����ᄃ����ܶ�Ϊag��cm-3����������� ��ֻҪ���г���ʽ����
��3����ͼ�п��Կ�����D��B�γɵ����ӻ�����ĵ���ʽΪ �������ӻ����ᄃ����ܶ�Ϊag��cm-3����������� ��ֻҪ���г���ʽ����
(1)H �������ڡ��ڢ�B�壻1s22s22p63s23p63d5����2������ HF��3��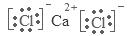
4��78g/mol/(6.02��1023��ag��cm-3)
������������������������֪����Ԫ�طֱ��ǣ�A��H��B��F��C��Cl��D��Ca��F��Mn����A��Ԫ�ط�����H��E��Ԫ�����ڱ��е�λ���ǵ������ڡ��ڢ�B�壻����+2�����ӵĵ����Ų�ʽΪ1s22s22p63s23p63d5����B���⻯��ľ��������Ƿ��Ӿ��壻F��Cl��ͬһ�����Ԫ�أ�����Ԫ�صķǽ�����F>Cl��Ԫ�صķǽ�����Խǿ�����⻯����ȶ��Ծ�Խǿ������B���⻯����C���⻯����ȣ����Ӽ��Խϴ����HF���� ��ͼ�п��Կ�������D��B�γɵ����ӻ������к���F��8������Ca:8��1/8+6��1/2=4,���Ի�ѧʽ��CaF2���û����������ӻ���������ʽΪ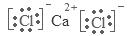 ;��ÿ�������к���4��CaF2�����ھ�����ܶ�
;��ÿ�������к���4��CaF2�����ھ�����ܶ�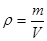 ������
������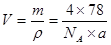 =4��78g/mol/(6.02��1023��ag��cm-3)��
=4��78g/mol/(6.02��1023��ag��cm-3)��
���㣺����Ԫ�ص��ƶϡ�Ԫ�������ڱ��е�λ�á�ԭ�ӵĺ�������Ų�ʽ������ʽ����д�����ʵ��ȶ��ԵıȽϡ���������ļ����֪ʶ��

������������ȷ����
| A��������Ǿ���ĸ������������Ƿ���й���ļ������� |
| B����������������ʸ������� |
| C�����塢�Ǿ�������й̶����۵� |
| D���ɲ����Ƴɹ���IJ����������˾�����Է��� |
�γ�������������Ե�ԭ���У�( )
��Ԫ������ ��ͬλ�� �ۻ�ѧ���ɼ���ʽ ��ͬ���칹���� ��ͬ����������
| A�����٢ڢ� | B�����ڢܢ� |
| C�����٢� | D���٢ڢۢܢ� |
��8�֣�ѡ������������д���пհף�
| A���������� | B������þ | C��He | D���������� |
��1�������д��ڷ��ӵ��� ����2�������м������Ӽ����й��ۼ����� ��
��3���ڻ�ʱ��Ҫ�ƻ����ۼ����� ����4����������ˮ�ķ��Ӿ����� ��
����˵�����ʾ������ȷ���ǣ� ��
| A�������ʵ���������������۷ֱ���ȫȼ�գ����߷ų������� |
| B����C(ʯī)��C(���ʯ) ��H=��11.9kJ��mol-1��֪�����ʯ��ʯī�ȶ� |
| C����25�棬101kPaʱ��2g������ȫȼ������Һ̬ˮ���ų�285.8kJ������������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ��2H2(g)��O2(g)=2H2O(l) ��H=��571.6kJ��mol-1 |
| D��H��(aq)��OH��(aq)= H2O(l) ��H=��57.3kJ��mol-1��������0.5molH2SO4��Ũ�����뺬1molNaOH������������Һ��ϣ��ų�����������57.3kJ |
��ѧ��ӦA2 �� B2 �� 2AB�������仯��ͼ��ʾ��������˵����ȷ����( )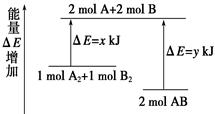
| A���÷�Ӧ�����ȷ�Ӧ |
| B������1 mol A��A����1 mol B��B���ܷų�x kJ������ |
| C������2 mol A��B����Ҫ����y kJ������ |
| D��2 mol AB������������1 mol A2��1 mol B2�������� |
���ʽṹ����ָ�������������н��������������ɵ���֮���ǿ�ҵ�����ã��н�������������Խǿ���������Ӳ��Խ���ۡ��е�Խ�ߣ��Ҿ��о�������һ��˵������ԭ�Ӱ뾶ԽС���۵�����Խ�࣬�������Խǿ���ɴ��ж�����˵��������ǣ� ����
| A��þ��Ӳ��С���� |
| B��þ���ۡ��е���ڸ� |
| C��þ��Ӳ�ȴ��ڼ� |
| D���Ƶ��ۡ��е���ڼ� |

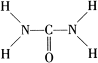 �� ���ؿ��������л����ʣ���Ҫ�������������������غ���������ѧʽΪ��Fe��H2NCONH2��6�ݣ�NO3��3��
�� ���ؿ��������л����ʣ���Ҫ�������������������غ���������ѧʽΪ��Fe��H2NCONH2��6�ݣ�NO3��3��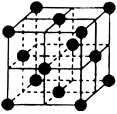
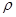 g��cm-3��NA��ʾ�����ӵ���������NaCl�������Ϊ cm3
g��cm-3��NA��ʾ�����ӵ���������NaCl�������Ϊ cm3