��Ŀ����
(14��)ijУ���л�ѧʵ�鿼��ʱ����ʦ����ͬѧ��һС����ɫ��ĩ�����ֺ�ɫ��ĩ����������ͭ��̿�ۻ����������ֵĻ�����ͬѧ��ͨ��ʵ��̽����ȷ����ijͬѧ̽���������£�
(1) ������裺
����1����ɫ��ĩ��̿�ۣ�
����2����ɫ��ĩ������ͭ��
����3��_________________________________________________________________��
(2) ���ʵ�鷽����
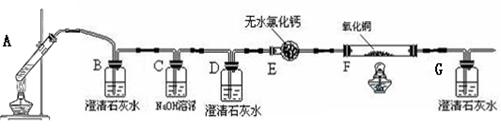
����ʵ��������������ͷ�����ȡ������ɫ��ĩ���ձ��У������������ϡ���ᡣ��ʵ���п��ܳ��ֵ��������Ӧ�������±�����������±���
|
ʵ���п��ܳ��ֵ����� |
���� |
|
�� |
����1���� |
|
�� |
����2���� |
|
�� |
����3���� |
(3) ����ʵ�飺ͨ��ʵ�鼰������ȷ���ú�ɫ����Ϊ̿�ۺ�����ͭ�Ļ���
(4) ��չ��Ϊ�˽�һ��̽��̿�ۺ�����ͭ�����ʣ������������ֻ���ﲹ����������ʵ�飬���˺�ɫ��ĩ������������(��̿��ȫ��Ӧ)������ȴ�����º�ȡʣ��������ձ��У��ټ������ϡ���ᣬ��Ӧ��ȫ���á���������и��⣺
���ձ���________(��С����ޡ�)�����
�������ú���Һ����ɫ�������Һ��������________(д��ѧʽ)��
�������ú���Һ����ɫ���÷�Ӧ�Ļ�ѧ����ʽΪ��
________________________________________________________________________��
��14�֣���ÿ��2�֣� (1)��ɫ��ĩ��̿�ۺ�����ͭ�Ļ���
(2)���ޱ仯������Һ����ɫ����ɫ��ĩ��ȫ�ܽ⡡����Һ����ɫ�����в��ֺ�ɫ������
(4)������H2SO4����CuO��H2SO4===CuSO4��H2O
����������1�����ݼ���1��2��֪������3Ӧ���Ǻ�ɫ��ĩ��̿�ۺ�����ͭ�Ļ���
��2��̼��ϡ�����Ӧ������ͭ��ϡ���ᷴӦ����������ͭ��ˮ����Һ������ɫ�����Զ�Ӧ������ֱ��Ǣ��ޱ仯������Һ����ɫ����ɫ��ĩ��ȫ�ܽ⡡����Һ����ɫ�����в��ֺ�ɫ�����
��4���������ڸ����������ȵ������£�̼�ܺ�����ͭ�����û���Ӧ������ͭ����ͭ��ϡ�����Dz���Ӧ�ģ������ձ��л��в����
�������ú���Һ����ɫ��˵��û������ͭ���ɣ�������������ᡣ
�������ú���Һ����ɫ��˵��������ͭ���ɣ���Ӧ�ķ���ʽΪCuO��H2SO4===CuSO4��H2O

 �㽭�¿γ���άĿ�������ʱ��ѵϵ�д�
�㽭�¿γ���άĿ�������ʱ��ѵϵ�д� ��������ϵ�д�
��������ϵ�д� ���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д� (14��)ijУ���л�ѧʵ�鿼��ʱ����ʦ����ͬѧ��һС����ɫ��ĩ�����ֺ�ɫ��ĩ����������ͭ��̿�ۻ����������ֵĻ�����ͬѧ��ͨ��ʵ��̽����ȷ����ijͬѧ̽���������£�
(1) ������裺
����1����ɫ��ĩ��̿�ۣ�
����2����ɫ��ĩ������ͭ��
����3��_________________________________________________________________��
(2) ���ʵ�鷽����
����ʵ��������������ͷ�����ȡ������ɫ��ĩ���ձ��У������������ϡ���ᡣ��ʵ���п��ܳ��ֵ��������Ӧ�������±�����������±���
| ʵ���п��ܳ��ֵ����� | ���� |
| �� | ����1���� |
| �� | ����2���� |
| �� | ����3���� |
(4) ��չ��Ϊ�˽�һ��̽��̿�ۺ�����ͭ�����ʣ������������ֻ���ﲹ����������ʵ�飬���˺�ɫ��ĩ������������(��̿��ȫ��Ӧ)������ȴ�����º�ȡʣ��������ձ��У��ټ������ϡ���ᣬ��Ӧ��ȫ���á���������и��⣺
���ձ���________(��С����ޡ�)�����
�������ú���Һ����ɫ�������Һ��������________(д��ѧʽ)��
�������ú���Һ����ɫ���÷�Ӧ�Ļ�ѧ����ʽΪ��
________________________________________________________________________��