题目内容
【题目】氯碱工业以电解精制饱和食盐水的方法制取氯气、氢气、烧碱和氯的含氧酸盐等系列化工产品。下图是离子交换膜法电解食盐水的示意图,图中的离子交换膜只允许阳离子通过。完成下列填空:

(1)电解饱和食盐水的化学方程式是_______________。
(2)离子交换膜的作用为:__________、___________。
(3)精制饱和食盐水从图中______位置补充,氢氧化钠溶液从图中_____位置流出(选填“a”、“b”、“c”或“d”)。
【答案】 2NaCl+2H2O![]() 2NaOH+H2↑+Cl2↑ 阻止OH-进入阳极室与Cl2发生副反应:2NaOH+Cl2==NaCl+NaClO+H2O 阻止阳极产生的Cl2和阴极产生的H2混合发生爆炸 a d
2NaOH+H2↑+Cl2↑ 阻止OH-进入阳极室与Cl2发生副反应:2NaOH+Cl2==NaCl+NaClO+H2O 阻止阳极产生的Cl2和阴极产生的H2混合发生爆炸 a d
【解析】(1)电解饱和食盐水的化学方程式是2NaCl+2H2O![]() 2NaOH+H2↑+Cl2↑。(2)由于阳极生成的氯气能与氢气或溶液中的氢氧根反应,所以离子交换膜的作用为阻止OH-进入阳极室与Cl2发生副反应:2NaOH+Cl2==NaCl+NaClO+H2O,同时也阻止阳极产生的Cl2和阴极产生的H2混合发生爆炸。(3)左侧是阳极区,氯离子放电,因此精制饱和食盐水从图中a位置补充;阴极区产生氢氧化钠,则氢氧化钠溶液从图中d位置流出。
2NaOH+H2↑+Cl2↑。(2)由于阳极生成的氯气能与氢气或溶液中的氢氧根反应,所以离子交换膜的作用为阻止OH-进入阳极室与Cl2发生副反应:2NaOH+Cl2==NaCl+NaClO+H2O,同时也阻止阳极产生的Cl2和阴极产生的H2混合发生爆炸。(3)左侧是阳极区,氯离子放电,因此精制饱和食盐水从图中a位置补充;阴极区产生氢氧化钠,则氢氧化钠溶液从图中d位置流出。

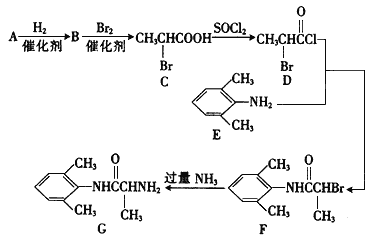
【题目】1,2-二氯丙烷(CH2ClCHClCH3)是重要的化工原料,工业上可用丙烯加成法生产,主要副产物为3-氯丙烯(CH2=CHCH2C1) ,反应原理为
i. CH2=CHCH,3(g)+Cl2(g)![]() CH2ClCHClCH3(g) ΔH1= -134 kJ mol-1
CH2ClCHClCH3(g) ΔH1= -134 kJ mol-1
ii. CH2=CHCH,3(g)+Cl2(g)![]() CH2=CHCH2Cl (g)+HCl(g) ΔH2= -l02 kJ mol-1
CH2=CHCH2Cl (g)+HCl(g) ΔH2= -l02 kJ mol-1
已知:相关化学键的键能数据如下表所示:
化学键 | C—C | C—C | C—Cl | Cl—Cl |
E/( kJ mol-1) | 611 | x | 328 | 243 |
请回答下列问题:
(1)由反应i计算出表中x=_____________。
(2)一定温度下,密闭容器中发生反应i和反应ii,达到平衡后增大压强,CH2C1CHC1CH3的产率____________(填“增大”“减小”或“不变”),理由是_________________________________。
(3)T1℃时,向10L恒容的密闭容器中充入1 mol CH2=CHCH2C1和2 mol HC1,只发生反应CH2=CH CH2Cl (g)+HCl(g)![]() CH2ClCHClCH3(g) ΔH3。5min反应达到平衡,测得 05 min内,用CH2ClCHClCH3表示的反应速率 v(CH2ClCHClCH3)=0.016 mol·L-1 min-1。
CH2ClCHClCH3(g) ΔH3。5min反应达到平衡,测得 05 min内,用CH2ClCHClCH3表示的反应速率 v(CH2ClCHClCH3)=0.016 mol·L-1 min-1。
①平衡时,HCl的体积分数为__________________(保留三位有效数字)。
②保持其它条件不变,6 min时再向该容器中充入0. 6 mol CH2=CHCH2Cl、0.2molHC1和0.1mol CH2ClCHClCH3,则起始反应速率 v正(HCl)______________ (填“>”“<”或“=”)V逆(HCl).
(4)一定压强下,向密闭容器中充入一定量的CH2=CHCH3和C12发生反应ii。设起始的![]() =w,平衡时Cl2的体积分数(φ)与温度(T)、w的关系如图甲所示。W=1时,正、逆反应的平衡常数(K)与温度(T)的关系如图乙所示。
=w,平衡时Cl2的体积分数(φ)与温度(T)、w的关系如图甲所示。W=1时,正、逆反应的平衡常数(K)与温度(T)的关系如图乙所示。
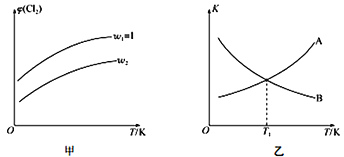
①图甲中,w2__________(填“>”“<”或“=”)1
②图乙中,表示正反应平衡常数的曲线为____________(填“A”或“B”),理由为________________。
③T1K下,平衡时a(Cl2)= ________________。
(5)起始时向某恒容绝热容器中充入1 mol CH2 =CHCH3和1 mol Cl2发生反应ii,达到平衡时,容器内气体压强_________________(填“增大”“减小”或“不变”)。