��Ŀ����
����08ɽ��ʵ����ѧ��ģ��11�֣�ABCDEF����������һ������������ͼ��ʾ���ת����ϵ�����з�Ӧ�����������Ѹ����Իش�
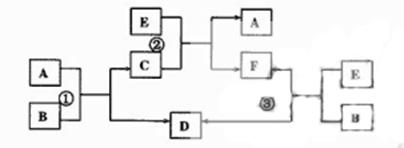
��1������Ӧ�٢ڢ۾�Ϊ��Һ�е��û���Ӧ��ADEΪ�����������ʣ���ADE�Ļ�ԭ����ǿ������˳��Ϊ__________����д����������Ҫ������ӷ���ʽ��
��Ӧ��________________________________________________��
��Ӧ��________________________________________________��
��2������Ӧ�٢ڢ۾�Ϊ���ֽⷴӦ��AE��Ϊ�������Һ���γɵ�D�ǰ�ɫ������д������Ҫ��Ļ�ѧ����ʽ��
��Ӧ��________________________________________________��
��Ӧ��________________________________________________��
��3����B��ˮ��C��һ���д��ԵĻ����E��һ����ɫ��ζ���ж����壬��Ӧ�ٵĻ�ѧ����ʽ��______��
������1��E>A>D����1�֣�
��Ӧ�٣�Fe+Cu2+====Fe2++Cu����Ӧ�ڣ�Zn+Fe2+====Zn2++Fe
����Ӧ�٣�Cu+2Ag+===Cu2++2Ag����Ӧ�ڣ�Fe+Cu2+===Fe2++Cu�ȣ�
������������Ҳ�ɣ�
��2����Ӧ�٣�3NH3?H2O+AlCl3===3NH4Cl+Al(OH)3��
��Ӧ�ڣ�NH4Cl+NaOH===NH3?H2O+NaCl
������������Ҳ�ɣ�
��3��3Fe+4H2O(g)====Fe3O4+4H2��ÿ��2�֣�

 һ����������ϵ�д�
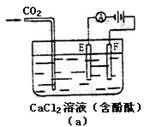
һ����������ϵ�д�(08ɽ��ʵ����ѧ���)��10�֣�����ͼ��a��Ϊ������ⱥ��CaCl2ˮ��Һ��װ�ã��Բ�Ϊ�缫��AΪ�������������һ��ʱ���ͼ��b��1Сʱ��CO2����ͨ����Һ�У�����ͼ��b�������ʵ�飨a���е�����ʱ��ı仯��ϵͼ�����ش��й����⡣
| |||
| |||
��1�����ʵ�飨a���е�����ʱ��ı仯��ϵͼ��
��2�����ʱF������ ��Ӧ���缫��ӦʽΪ ��
E���ĵ缫��ӦʽΪ ___________ �������ܻ�ѧ����ʽΪ ��
��3���������������
�� �� ��
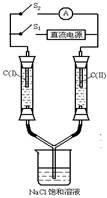 ��08ɽ��ʵ����ѧ��13������ͼ��ʾװ�ã�����������ʢ�����з�̪��Һ��NaCl������Һ��C����C����Ϊ���ʯī�缫����ͨS1��C��������Һ��죬�������������������ɡ�һ��ʱ�������������Һ��δ����缫�����Ͽ�S1����ͨS2����������ָ�뷢��ƫת,˵����ʱ��װ���γ���ԭ��أ����ڸ�ԭ��ص�������ȷ����
��08ɽ��ʵ����ѧ��13������ͼ��ʾװ�ã�����������ʢ�����з�̪��Һ��NaCl������Һ��C����C����Ϊ���ʯī�缫����ͨS1��C��������Һ��죬�������������������ɡ�һ��ʱ�������������Һ��δ����缫�����Ͽ�S1����ͨS2����������ָ�뷢��ƫת,˵����ʱ��װ���γ���ԭ��أ����ڸ�ԭ��ص�������ȷ����