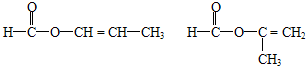��Ŀ����
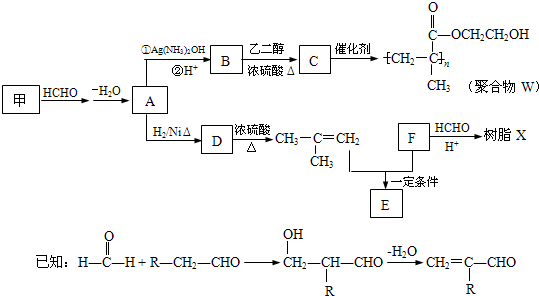
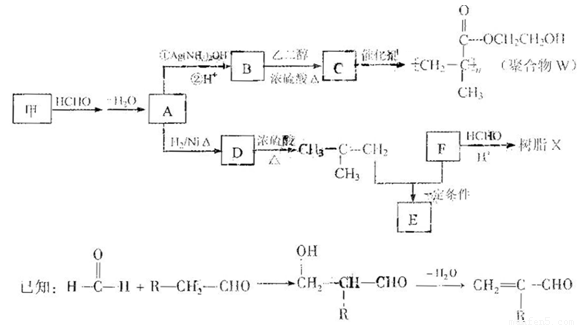
���������۾�����W����֬X�ĺϳ�·�����£�
��1��A �к��еĹ�����������
��2��B��C��Ӧ�Ļ�ѧ����ʽ��
��3��B�ж���ͬ���칹�壮�������Һ���̼̼˫����ͬ���칹�干��
��4��Ϊȷ��ij�����廯����F�Ľṹ������������ʵ�飺
�پ������Dzⶨ�����л������Է�������Ϊ110
��ȷ��ȡ���л�����Ʒ5.50g�����ȼ�պ����ɱ�״����6.72LCO2��2.70gH2O��
����˴Ź�����ͼ��ʾ��F��2�����շ壬ǿ��Ϊ2��1
��F�Ľṹ��ʽΪ

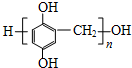
��5��д����֬X���ܵĽṹ��ʽ��д��һ�ּ��ɣ�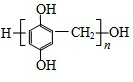
 ��
��
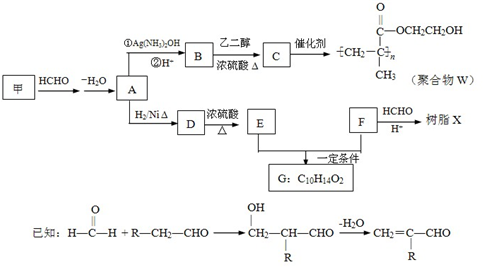
��6��G������������˴Ź�����ͼ����4�����շ壮д������E+F��G�Ļ�ѧ����ʽ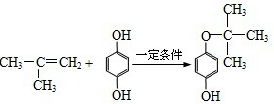
 ��
��

��1��A �к��еĹ�����������
̼̼˫����ȩ��
̼̼˫����ȩ��
����2��B��C��Ӧ�Ļ�ѧ����ʽ��
CH2=C��CH3��COOH+HOCH2CH2OH
CH2=C��CH3��COOCH2CH2OH+H2O
| Ũ���� |
| �� |
CH2=C��CH3��COOH+HOCH2CH2OH
CH2=C��CH3��COOCH2CH2OH+H2O
��| Ũ���� |
| �� |
��3��B�ж���ͬ���칹�壮�������Һ���̼̼˫����ͬ���칹�干��
5
5
�֣�������˳���칹����ͬ����д�������ܷ���������Ӧ���Һ��м�������ͬ���칹��Ľṹ��ʽ��HCOOCH=CHCH3��HCOOC��CH3��=CH2
HCOOCH=CHCH3��HCOOC��CH3��=CH2
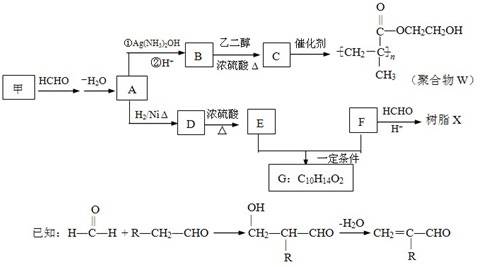
����4��Ϊȷ��ij�����廯����F�Ľṹ������������ʵ�飺
�پ������Dzⶨ�����л������Է�������Ϊ110
��ȷ��ȡ���л�����Ʒ5.50g�����ȼ�պ����ɱ�״����6.72LCO2��2.70gH2O��
����˴Ź�����ͼ��ʾ��F��2�����շ壬ǿ��Ϊ2��1
��F�Ľṹ��ʽΪ


��5��д����֬X���ܵĽṹ��ʽ��д��һ�ּ��ɣ�


��6��G������������˴Ź�����ͼ����4�����շ壮д������E+F��G�Ļ�ѧ����ʽ


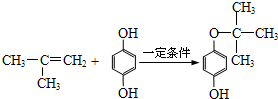
����������Ϣ��֪����Ϊȩ����HCHO��Ӧ������ˮ����A����A�к���C=C��-CHO���ɾۺ���W�Ľṹ��֪��AΪ2����ϩȩ��BΪ2-����ϩ�ᣬB���Ҷ�������������Ӧ����C��CΪCH2=C��CH3��COOCH2CH2OH��A��D�����ӳɷ�Ӧ��DΪ2��������D��E����������ȥ��Ӧ����EΪ2����ϩ����E+F��Ӧ����G����̼ԭ����Ŀ��֪��FΪ��6��̼ԭ�ӵĶԶ����ӣ�Ȼ�����л���Ľṹ�����������
����⣺����Ϣ��֪����Ϊȩ����HCHO��Ӧ������ˮ����A����A�к���C=C��-CHO���ɾۺ���W�Ľṹ��֪��AΪ2����ϩȩ��BΪ2-����ϩ�ᣬB���Ҷ�������������Ӧ����C��CΪCH2=C��CH3��COOCH2CH2OH��A��D�����ӳɷ�Ӧ��DΪ2��������D��E����������ȥ��Ӧ����EΪ2����ϩ����E+F��Ӧ����G����̼ԭ����Ŀ��֪��FΪ��6��̼ԭ�ӵĶԶ����ӣ�
��1��AΪ2����ϩȩ������̼̼˫����ȩ�����ʴ�Ϊ��̼̼˫����ȩ����
��2��B��CΪ������Ӧ����Ӧ�Ļ�ѧ����ʽΪCH2=C��CH3��COOH+HOCH2CH2OH
CH2=C��CH3��COOCH2CH2OH+H2O��
�ʴ�Ϊ��CH2=C��CH3��COOH+HOCH2CH2OH
CH2=C��CH3��COOCH2CH2OH+H2O��
��3��BΪ2-����ϩ�ᣬ�������Һ���̼̼˫����ͬ���칹��ΪHCOOCH=CHCH3��HCOOC��CH3��=CH2��CH3COOCH=CH2��CH2=CHCOOCH3��HCOOCH2CH=CH2����5�֣������ܷ���������Ӧ���Һ��м�������ͬ���칹��Ľṹ��ʽΪHCOOCH=CHCH3��HCOOC��CH3��=CH2���ʴ�Ϊ��5��HCOOCH=CHCH3��HCOOC��CH3��=CH2��
��4���پ������Dzⶨ�����л������Է�������Ϊ110����ȷ��ȡ���л�����Ʒ5.50g�����ȼ�պ����ɱ�״����6.72LCO2��2.70gH2O����C��Hԭ�Ӹ���֮��Ϊ1��1��
����˴Ź�����ͼ��ʾ��F��2�����շ壬ǿ��Ϊ2��1����ṹ�д�������λ�õ�Hԭ�ӣ�����FΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��5��F��HCO��Ӧ����X�������Ϣ��֪�������۷�Ӧ����֬X���ܵĽṹ��ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��6��G������������˴Ź�����ͼ����4�����շ壬��G����4��H������E+F��G�Ļ�ѧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��1��AΪ2����ϩȩ������̼̼˫����ȩ�����ʴ�Ϊ��̼̼˫����ȩ����
��2��B��CΪ������Ӧ����Ӧ�Ļ�ѧ����ʽΪCH2=C��CH3��COOH+HOCH2CH2OH
| Ũ���� |
| �� |
�ʴ�Ϊ��CH2=C��CH3��COOH+HOCH2CH2OH
| Ũ���� |
| �� |
��3��BΪ2-����ϩ�ᣬ�������Һ���̼̼˫����ͬ���칹��ΪHCOOCH=CHCH3��HCOOC��CH3��=CH2��CH3COOCH=CH2��CH2=CHCOOCH3��HCOOCH2CH=CH2����5�֣������ܷ���������Ӧ���Һ��м�������ͬ���칹��Ľṹ��ʽΪHCOOCH=CHCH3��HCOOC��CH3��=CH2���ʴ�Ϊ��5��HCOOCH=CHCH3��HCOOC��CH3��=CH2��
��4���پ������Dzⶨ�����л������Է�������Ϊ110����ȷ��ȡ���л�����Ʒ5.50g�����ȼ�պ����ɱ�״����6.72LCO2��2.70gH2O����C��Hԭ�Ӹ���֮��Ϊ1��1��
����˴Ź�����ͼ��ʾ��F��2�����շ壬ǿ��Ϊ2��1����ṹ�д�������λ�õ�Hԭ�ӣ�����FΪ
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����5��F��HCO��Ӧ����X�������Ϣ��֪�������۷�Ӧ����֬X���ܵĽṹ��ʽΪ
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����6��G������������˴Ź�����ͼ����4�����շ壬��G����4��H������E+F��G�Ļ�ѧ����ʽΪ
 ��
���ʴ�Ϊ��
 ��
�����������⿼���л�����ƶϣ�������֪��Ϣ���л���Ľṹ�������ŵı仯��̼ԭ����Ŀ�ı仯�ƶϸ������ǽ����Ĺؼ���ע������л���Ľṹ�����ʣ���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ