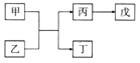
��Ŀ����
����Ŀ��X��Y��Z��W��U����Ԫ�أ���λ�����ڱ���ǰ�����ڣ����ǵĺ˵�����������ӣ��Һ˵����֮��Ϊ57��Yԭ�ӵ�L��p�������2�����ӣ�Z��ԭ�Ӻ���������δ�ɶԵ��ӣ�W��Yԭ�ӵļ۵�������ͬ��Uԭ�ӵ�K���������������������Ϊ2:1����d�������ȫ����״̬��
(1)Uԭ�ӵĵ����Ų�ʽΪ______________��
(2)Y��W�縺�ԣ���С����______(��Ԫ�ط��ţ���Y��Z�ĵ�һ�����ܣ��ϴ����______(��Ԫ�ط��ţ���
(3)X��Z�γ��������Qʱ��Zԭ�Ӳ�ȡ���ӻ��������Ϊ_______��Q���ӿռ乹��Ϊ_________����д��U2+��Q�γ������ӵ����ӷ�Ӧ����ʽ��_______________��
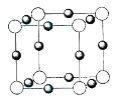
(4)U+��Z3+�γɵľ����ṹ��ͼ��ʾ�����������Ӽ�˼��Ϊa cm��ͬ�D��Z3+������U+��_____�����þ�����ܶ�Ϊ______g cm-3(NA ��ʾ�����ӵ�����)��
���𰸡� 1s22s22p63s23p63d104s1 Si N sp3 ������ Cu2++4NH3 = [Cu(NH3)4]2+ 6 ![]() ��
��![]()
�����������������������Ҫ����ԭ�ӽṹ�����ӽṹ�;���ṹ��
Yԭ�ӵ�L��p�������2�����ӣ�Y��̼��W��Yԭ�ӵļ۵�������ͬ��W�ǹ衣Z��ԭ�Ӻ���������δ�ɶԵ��ӣ�Z�ǵ��� Uԭ�ӵ�K���������������������Ϊ2:1����d�������ȫ����״̬��U��ͭ��X��Y��Z��W��U�ĺ˵����֮��Ϊ57��X������
(1)Uԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d104s1��
(2)ͬ����Ԫ�صĵ縺�ԣ����ϵ�����С��Y��W�縺�ԣ���С����Si��ͬ����Ԫ�أ������ң���һ��������������Y��Z�ĵ�һ�����ܣ��ϴ����N��
(3)X��Z�γ��������Q�ǰ���Zԭ�ӵļ۲���Ӷ���4����ȡ���ӻ��������Ϊsp3��Q���ӿռ乹��Ϊ�����Σ�U2+��Q�γ������ӷ�Ӧ�ĵ����ӷ���ʽ��Cu2++4NH3 = [Cu(NH3)4]2+ ��
(4)��ͬ�D��Z3+������U+��6�����þ�����ܶ�Ϊ![]() g cm-3��
g cm-3��

����Ŀ���о�CO2ת�����л���ʵ��̼ѭ����ʵ�����Ŀɳ�����չ������Ҫ�����塣��ȼú�����е�CO2ת��Ϊ�����ѵķ�Ӧԭ��Ϊ2CO2(g)+6H2(g)![]() CH3OCH3(g)+3H2O(1)��
CH3OCH3(g)+3H2O(1)��
(1)��֪һ��ѹǿ�£��÷�Ӧ���¶�����ʱ��CH3OCH3(g)��Ũ�ȼ�С����Ӧ���ʱ��H_____0���ر��S_______0(�ֱ��>������<������=��)��
(2)��ͬ�����£������Ϊ2L���ܱ�������ѡ�ò�ͬ�Ĵ����������������CH3OCH3������ʱ��仯��ͼ��ʾ��

��ͼ��0-4min��A�ķ�Ӧ����v(CO2)=________�����¶���ƽ�ⳣ���ı���ʽΪ________��
�������й�˵����ȷ����______(����ĸ���)��
A.��Ӧ�Ļ�ܴ�С˳���ǣ�Ea(A)<Ea(B)< Ea(C)
B.�����¶���ʹ��Ӧ���ʼӿ죬����Ϊ����Ӱٷ�������
C.��λʱ��������CO2������H2����ĿΪ3:1ʱ��˵����Ӧ�Ѿ��ﵽƽ��
D.����ѹǿ��ƽ�������ƶ���ƽ�ⳣ��Kֵ����
(3)ij�¶��£�������ɱ���ܱ������У��ı���ʼʱ�����ʵ���ʼͶ�������ڲ�ͬ��ѹǿ�£�ƽ��ʱ�����������CH3OCH3(g)�����ʵ������±���ʾ��
��� | ��ʼͶ������ͬ��ѹǿ�£� ƽ��CH3OCH3(g)������ͬ��ѹǿ | P1 | P2 | P3 |
I | 2.0 molCO2 6.0molH2 | 0.10mol | 0.04 mol | 0.02mol |
II | 1.0molCO2 3.0molH2 | X1 | Y1 | Z1 |
III | 1.0mol CH3OCH3 3.0mol H2O | X2 | Y2 | Z2 |
��P1___P2(� >������<����=��)���ж�����Ϊ____________��
��X1=______________��
��P2�£�III��CH3OCH3��ƽ��ת����Ϊ_____________��