��Ŀ����
ʵ�����Ʊ�1��2-��������ķ�Ӧԭ�����£�
CH3CH2OH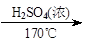 CH2=CH2+H2O
CH2=CH2+H2O
CH2=CH2+Br2��BrCH2CH2Br
���ܴ��ڵ���Ҫ����Ӧ�У��Ҵ���Ũ����Ĵ�������140����ˮ�������ѣ�Ũ������Ҵ�����ΪCO2�ȡ�
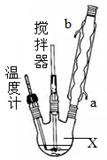
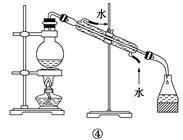
������������������Ҵ��Ʊ�1��2-���������װ������ͼ��ʾ��
����������:
�ش��������⣺
��1��Aװ���Ϸ�ʹ�õ�Һ©�����ŵ��ǣ�_________________________���������һ��ʱ��������ǼӴ�Ƭ��Ӧ�ò�ȡ����ȷ������_____________________(����ȷ�𰸱��)��
A���������� B����ȴ�� C�����貹�� D����������
��2��Bװ�õ�������_____________________________________��
��3����װ��C��Ӧ����________(����ȷѡ��ǰ����ĸ)����Ŀ����______________��
a��ˮ b��Ũ���� c������������Һ d������̼��������Һ
��4���жϸ��Ʊ���Ӧ�Ѿ��������������____________________________��
��5��Dװ�þ�֧�Թ���������ˮ����Һ��(�ٶ�������ͬ)���������ŵ�________________��
��6����Ӧ������Ӧ����ˮ��ȴװ��D������ҪĿ����_____________�����ֲ��ܹ�����ȴ(���ñ�ˮ)����ԭ����_________________________��
CH3CH2OH
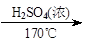 CH2=CH2+H2O
CH2=CH2+H2OCH2=CH2+Br2��BrCH2CH2Br
���ܴ��ڵ���Ҫ����Ӧ�У��Ҵ���Ũ����Ĵ�������140����ˮ�������ѣ�Ũ������Ҵ�����ΪCO2�ȡ�
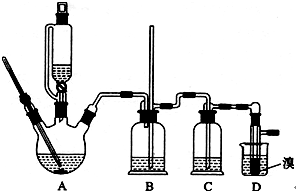
������������������Ҵ��Ʊ�1��2-���������װ������ͼ��ʾ��

����������:
| | �Ҵ� | 1,2-�������� | ���� |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶ�/g��cm3 | 0��79 | 2��2 | 0��71 |
| �е�/oC | 78��5 | 132 | 34��6 |
| �۵�/oC | -130 | 9 | -116 |
��1��Aװ���Ϸ�ʹ�õ�Һ©�����ŵ��ǣ�_________________________���������һ��ʱ��������ǼӴ�Ƭ��Ӧ�ò�ȡ����ȷ������_____________________(����ȷ�𰸱��)��
A���������� B����ȴ�� C�����貹�� D����������
��2��Bװ�õ�������_____________________________________��
��3����װ��C��Ӧ����________(����ȷѡ��ǰ����ĸ)����Ŀ����______________��
a��ˮ b��Ũ���� c������������Һ d������̼��������Һ
��4���жϸ��Ʊ���Ӧ�Ѿ��������������____________________________��
��5��Dװ�þ�֧�Թ���������ˮ����Һ��(�ٶ�������ͬ)���������ŵ�________________��
��6����Ӧ������Ӧ����ˮ��ȴװ��D������ҪĿ����_____________�����ֲ��ܹ�����ȴ(���ñ�ˮ)����ԭ����_________________________��
��1������©���ڵ�Һ����˳������(1��)��B(1��)
��2��ƽ��ѹǿ�����װ���Ƿ�������(1��)
��3��c(1��)�����շ�Ӧ���ɵ�SO2����������(1��)
��4�������ɫ��ȫ��ȥ(1��)
��5������1��2-���������ˮ�������ֲܷ㣬ˮ���ϲ�����Һ�⣬��ֹ��Ʒ�ӷ�������(2��)
��6����ϩ���嵥�ʷ�Ӧ�ų���������ȴ���Ա�����Ĵ����ӷ�(1��)��1��2-����������۵�Ϊ9�棬������ȴ���ɹ����ʹ�������ܶ���(2��)
��2��ƽ��ѹǿ�����װ���Ƿ�������(1��)
��3��c(1��)�����շ�Ӧ���ɵ�SO2����������(1��)
��4�������ɫ��ȫ��ȥ(1��)
��5������1��2-���������ˮ�������ֲܷ㣬ˮ���ϲ�����Һ�⣬��ֹ��Ʒ�ӷ�������(2��)
��6����ϩ���嵥�ʷ�Ӧ�ų���������ȴ���Ա�����Ĵ����ӷ�(1��)��1��2-����������۵�Ϊ9�棬������ȴ���ɹ����ʹ�������ܶ���(2��)
�����������1��Aװ���Ϸ�ʹ�õ�Һ©�����ŵ��DZ���©���ڵ�Һ����˳�����£��������һ��ʱ��������ǼӴ�Ƭ��Ӧ�ò�ȡ����ȷ��������ȴ�ӡ�
��2��Bװ�õ�������ƽ��ѹǿ�����װ���Ƿ���������
��3����װ��C��Ӧ��������������Һ����Ŀ�������շ�Ӧ���ɵ�SO2���������壬��ΪSO2�ܱ���������
��4���жϸ��Ʊ���Ӧ�Ѿ�������������������ɫ��ȫ��ȥ��
��5��Dװ�þ�֧�Թ���������ˮ����Һ��(�ٶ�������ͬ)������1��2-���������ˮ�������ֲܷ㣬ˮ���ϲ�����Һ�⣬��ֹ��Ʒ�ӷ������á�
��6����Ӧ������Ӧ����ˮ��ȴװ��D������ҪĿ������ϩ���嵥�ʷ�Ӧ�ų���������ȴ���Ա�����Ĵ����ӷ������ֲ��ܹ�����ȴ(���ñ�ˮ)����ԭ����1��2-����������۵�Ϊ9�棬������ȴ���ɹ����ʹ�������ܶ�����

��ϰ��ϵ�д�
�����Ŀ
 CO(NH2)2��H2O2�����������صIJ����������£�
CO(NH2)2��H2O2�����������صIJ����������£�