��Ŀ����
ij������A������ˮ���õ�B���ữ��õ�C��C8H8O2�����˴Ź������ױ�����C���б������ұ�������2����ԭ�ӡ�B�������и�����Ӧ��õ�G��C8H12O4�����˴Ź���������ʾG��ֻ��1����ԭ�ӡ�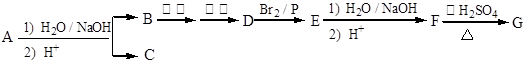
��֪��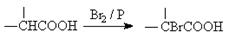
��ش��������⣺
��1���л������A��������______�࣬C��������________�ࡣ
��2��д����������������C������ͬ���칹�壺__________________________________________��
���DZ��Ķ�λ��ȡ�������������FeCl3��Һ������ɫ��Ӧ���۲�����ϩ����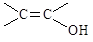 ���ṹ��
���ṹ��
��3��д��F��G��Ӧ�Ļ�ѧ����ʽ��_____________________________________________________��
��4��д��A�Ľṹ��ʽ��_____________________________________��
��5����G�Ĵֲ����У�����⺬�оۺ������ʡ�д���ۺ������ʿ��ܵĽṹ��ʽ����Ҫ��д��1�֣���__________________________________��
������12�֣���1����2�֣��� ���� ��2����4�֣�
��3����2�֣� 
��4����2�֣� ��5����2�֣�
��5����2�֣�
�������������������A������ˮ�⡢�ữ��õ�B��C��C8H8O2����B����������������D����˵��B�Ǵ���DA�����ᡣ���A������C�����ᡣC��C8H8O2���ĺ˴Ź������ױ������б������ұ�������2����ԭ�ӣ���˵����������2��ȡ������������ȡ�������ڶ�λ������C�ķ���ʽ֪��C�ǶԼ������ᣬ�ṹ��ʽ�� ��D���塢P��Ӧ����E�����������Ϣ֪��E�Ǻ�����ԭ�ӵ����ᣬE����ˮ����ữ������F��F�Ǻ����ǻ������ᡣ����F����G�ķ�Ӧ������֪���÷�Ӧ���ǻ�����ȥ��Ӧ��������Ӧ������G�Ļ�ѧʽ�Լ��˴Ź���������ʾG��ֻ��1����ԭ�ӿ�֪���÷�Ӧ���������ǻ�����ȥ��Ӧ��Ӧ�����ǻ����Ȼ���������Ӧ�������ɵ�G�ǻ���������G�Ľṹ��ʽֻ����
��D���塢P��Ӧ����E�����������Ϣ֪��E�Ǻ�����ԭ�ӵ����ᣬE����ˮ����ữ������F��F�Ǻ����ǻ������ᡣ����F����G�ķ�Ӧ������֪���÷�Ӧ���ǻ�����ȥ��Ӧ��������Ӧ������G�Ļ�ѧʽ�Լ��˴Ź���������ʾG��ֻ��1����ԭ�ӿ�֪���÷�Ӧ���������ǻ�����ȥ��Ӧ��Ӧ�����ǻ����Ȼ���������Ӧ�������ɵ�G�ǻ���������G�Ľṹ��ʽֻ���� �����F��2-��2-�ǻ����ᣬ��ṹ��ʽ��(CH3)2COHCOOH��E��2-��2-����ᣬ��ṹ��ʽ��(CH3)2CBrCOOH��D��2-�����ᣬ��ṹ��ʽ��(CH3)2CHCOOH������B��2-����������ṹ��ʽ��(CH3)2CHCH2OH����A�Ľṹ��ʽ��
�����F��2-��2-�ǻ����ᣬ��ṹ��ʽ��(CH3)2COHCOOH��E��2-��2-����ᣬ��ṹ��ʽ��(CH3)2CBrCOOH��D��2-�����ᣬ��ṹ��ʽ��(CH3)2CHCOOH������B��2-����������ṹ��ʽ��(CH3)2CHCH2OH����A�Ľṹ��ʽ�� ��
��
��1��ͨ�����Ϸ���֪��A���ʷ����к����������������ࣻC���ʷ����к����Ȼ������������ࡣ
��2�����DZ��Ķ�λ��ȡ�������˵�������Ϻ���2��ȡ������������FeCl3��Һ������ɫ��Ӧ��˵������һ��ȡ�����Ƿ��ǻ����۲�����ϩ����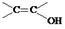 ���ṹ����������������C������ͬ���칹��Ľṹ��ʽ��
���ṹ����������������C������ͬ���칹��Ľṹ��ʽ�� ��
��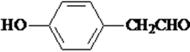 ��
�� ��
�� ��
��
��3��F��G��������Ӧ����˷�Ӧ�Ļ�ѧ����ʽΪ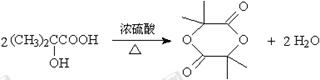 ��
��
��4��A�Ľṹ��ʽ ��
��
��5������F�к����ǻ����Ȼ������Ի��п��ܷ������۷�Ӧ�γɸ߷��ӻ������˾ۺ������ʿ��ܵĽṹ��ʽΪ ��
��
���㣺�����л����ƶϡ��л�����ࡢͬ���칹���Լ�����ʽ����д��

��ϩ������ʯ�͵���Ҫ�л�����ԭ�ϣ������ͨ����������һ�����ҵ�ʯ�ͻ�����չˮƽ���������·�ش�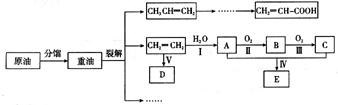
��֪��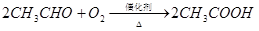
��1����ӦII�Ļ�ѧ����ʽ�� ��
��2��DΪ�߷��ӻ������������������ְ�װ���ϣ���ṹ��ʽ�� ��
��3��E������ζ�����ʣ���ʵ��������ͼװ����ȡ��
�ٷ�ӦIV�Ļ�ѧ����ʽ�� ���÷�Ӧ����Ϊ ��
�ڸ�װ��ͼ����һ�����ԵĴ����� ��
��4��Ϊ��֤��Ũ�����ڷ�ӦIV�����˴�������ˮ�������ã�ijͬѧ������ͼ�Ľ���װ�ý���������4��ʵ�顣ʵ�鿪ʼ���þƾ�����3min���ټ���ʹ֮����3min��ʵ�����������С�Թ����ٲ��л���ĺ�ȣ�ʵ���¼���£�
| ʵ���� | �Թܼ����Լ� | �Թ������Լ� | �л���ĺ��/cm |
| A | 2 mL�Ҵ���1 mL���ᡢ 1mL18mol��L��1Ũ���� | ����Na2CO3��Һ | 3.0 |
| B | 2 mL�Ҵ���1 mL���� | 0.1 | |
| C | 2 mL�Ҵ���1 mL���ᡢ 3 mL 2mol��L��1 H2SO4 | 0.6 | |
| D | 2 mL�Ҵ���1 mL���ᡢ���� | 0.6 |
��ʵ��D��Ŀ������ʵ��C����գ�֤��H+��������Ӧ���д����á�ʵ��D��Ӧ��������������Ũ�ȷֱ���3mL�� mol��L��1��
�ڷ���ʵ�� ����ʵ���ţ������ݣ������Ʋ��ŨH2SO4����ˮ����������������IJ��ʡ�
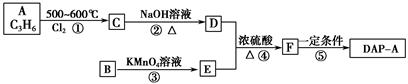
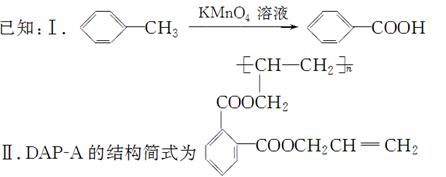
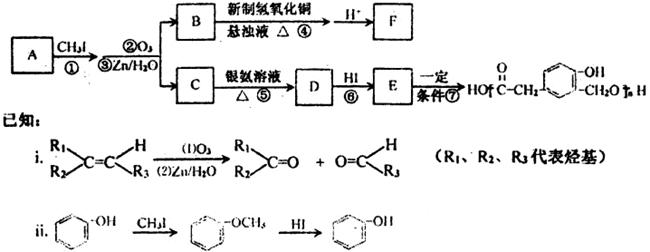
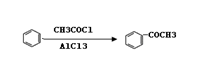
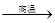 CO��+H2�������ᱽ����(F)���������Ϻ�ҩ�����Ҫԭ�ϡ���ͼ����úΪԭ�Ϻϳɼ��ᱽ������·��ͼ�����ַ�Ӧ����������������ȥ������D�ķ���ʽΪC9H10O�����ܷ���������Ӧ��
CO��+H2�������ᱽ����(F)���������Ϻ�ҩ�����Ҫԭ�ϡ���ͼ����úΪԭ�Ϻϳɼ��ᱽ������·��ͼ�����ַ�Ӧ����������������ȥ������D�ķ���ʽΪC9H10O�����ܷ���������Ӧ��

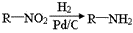
 Ϊԭ�Ϻϳ�
Ϊԭ�Ϻϳ� 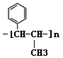 ����ĵ�һ���ĺϳ�·�ߺ����һ���Ļ�ѧ����ʽ�����Լ���ѡ����
����ĵ�һ���ĺϳ�·�ߺ����һ���Ļ�ѧ����ʽ�����Լ���ѡ���� ��1��������D�й����ŵ�����Ϊ �� ��������
��1��������D�й����ŵ�����Ϊ �� ��������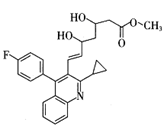 ����ת��ΪF������Ϊ�ϳ�·������Ʋ���ڵ�Ŀ���� ��
����ת��ΪF������Ϊ�ϳ�·������Ʋ���ڵ�Ŀ���� �� ���÷�Ӧԭ�����л��ϳ��о��й㷺Ӧ�á���д����
���÷�Ӧԭ�����л��ϳ��о��й㷺Ӧ�á���д���� Ϊ��Ҫԭ���Ʊ�
Ϊ��Ҫԭ���Ʊ�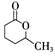 �ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ�����£�CH3CH2OH
�ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ�����£�CH3CH2OH CH2��CH2
CH2��CH2 CH3CH3
CH3CH3
 ��������
��������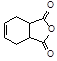 �Ǻϳɿ�����ҩ������Τ���м��壬����ƺ���������
�Ǻϳɿ�����ҩ������Τ���м��壬����ƺ��������� ��
�� Ϊԭ�Ϻϳɸû�����úϳ�·������ͼ��ʾ����ע����Ӧ���������ϳ�·������ͼʾ�����£�
Ϊԭ�Ϻϳɸû�����úϳ�·������ͼ��ʾ����ע����Ӧ���������ϳ�·������ͼʾ�����£� 

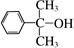 Ҳ����������Ʒ�Ӧ�۵ķ�Ӧ�����л���������Ľṹ��ʽ��______________��
Ҳ����������Ʒ�Ӧ�۵ķ�Ӧ�����л���������Ľṹ��ʽ��______________��