��Ŀ����
��12�֣���ҵ�����������ᣨ�е㣺90oC��ʱ��ͬʱ�������������ƣ��乤���������£�

��1����ȴ���˵�Ŀ���ǽ���NaHSO4�� �������NaHSO4���塣
��2����Ӧ��2�з�����Ӧ�����ӷ���ʽΪ ��SO2���������� ����
��3��ѭ��ʹ�õ������� ��
��4������ͨ��������Һ�ķ����õ��������ԭ������� ��
��5����ҵ���ò���������ͭ�����������������Ҳ���Ƶø����ᣬ���������ɵõ�20%�ĸ����ᡣд�������ĵ缫��Ӧʽ������������������Ի�ѧʽ���֣� ��

��1����ȴ���˵�Ŀ���ǽ���NaHSO4�� �������NaHSO4���塣
��2����Ӧ��2�з�����Ӧ�����ӷ���ʽΪ ��SO2���������� ����
��3��ѭ��ʹ�õ������� ��
��4������ͨ��������Һ�ķ����õ��������ԭ������� ��
��5����ҵ���ò���������ͭ�����������������Ҳ���Ƶø����ᣬ���������ɵõ�20%�ĸ����ᡣд�������ĵ缫��Ӧʽ������������������Ի�ѧʽ���֣� ��
��12�֣���1���ܽ�ȣ�2�֣���2��2ClO2��SO2��4OH����2ClO2����SO42����2H2O����2�֣���ԭ��2�֣�
��3��H2SO4��2�֣� ��4��������ķе�Ƚϵͣ����״���Һ���ݳ�����2�֣�
��5��������Ӧʽ HCl+ 4H2O -8e��= HClO4 + 8H+����2�֣�
��3��H2SO4��2�֣� ��4��������ķе�Ƚϵͣ����״���Һ���ݳ�����2�֣�
��5��������Ӧʽ HCl+ 4H2O -8e��= HClO4 + 8H+����2�֣�
��1���ܽ�� NaHSO4���ܽ�����¶ȵ����߶��������¶ȵĽ��Ͷ���С����ȴ���������NaHSO4���塣
��2��2ClO2��SO2��4OH����2ClO2����SO42����2H2O
�ڷ�Ӧ���ɵ�SO2��Ԫ����+4�۵�+6�ۣ�ʧȥ���ӣ�Ϊ��ԭ��
��4������ʱ�����ʰ��е��ɵ͵������α��������������ķе�Ϊ90 oC������������
��5�����ݵ���ԭ������������ʧȥ���ӣ����ϼ����ߣ�������
��2��2ClO2��SO2��4OH����2ClO2����SO42����2H2O
�ڷ�Ӧ���ɵ�SO2��Ԫ����+4�۵�+6�ۣ�ʧȥ���ӣ�Ϊ��ԭ��
��4������ʱ�����ʰ��е��ɵ͵������α��������������ķе�Ϊ90 oC������������
��5�����ݵ���ԭ������������ʧȥ���ӣ����ϼ����ߣ�������

��ϰ��ϵ�д�
�����Ŀ
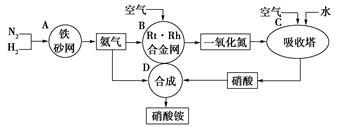
 (1)������C��ͨ�˿�����Ŀ����
(1)������C��ͨ�˿�����Ŀ����  2NH3(g)����H=-92kJ��mol��Ϊ���������ת���ʣ��˲�ȡ�Ĵ�ʩ�� ��(����ĸ)
2NH3(g)����H=-92kJ��mol��Ϊ���������ת���ʣ��˲�ȡ�Ĵ�ʩ�� ��(����ĸ)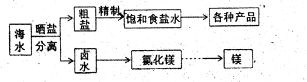
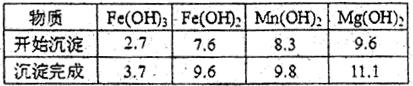
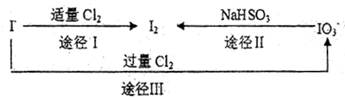

 ��
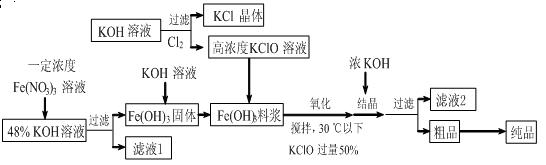
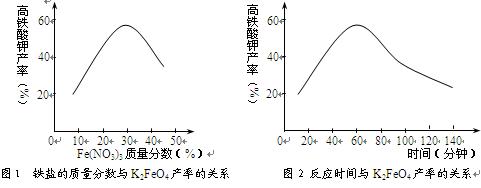
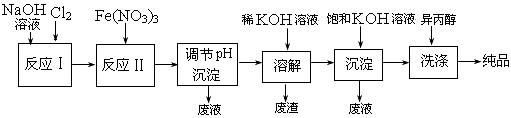
��
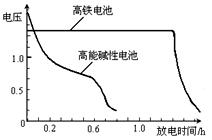
 ����ͼΪ�õ�غͳ��õĸ��ܼ��Ե�صķŵ����ߣ��ɴ˿ɵó��ĸ�����ص��ŵ��������� �������� ��
����ͼΪ�õ�غͳ��õĸ��ܼ��Ե�صķŵ����ߣ��ɴ˿ɵó��ĸ�����ص��ŵ��������� �������� ��