��Ŀ����
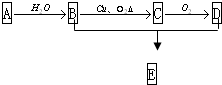 ��ͼ��ʾ����֪A�IJ�����һ������ʯ�ͻ���ˮƽ�ı�־��D�������ԣ�E�Ǿ�����ζ�IJ�����ˮ��Һ�壮
��ͼ��ʾ����֪A�IJ�����һ������ʯ�ͻ���ˮƽ�ı�־��D�������ԣ�E�Ǿ�����ζ�IJ�����ˮ��Һ�壮��1��д��D�Ĺ��������ƣ�
��2��A��B�Ļ�ѧ��Ӧ����ʽ��
| һ�������� |
| һ�������� |
��3��д��B+D��E�Ļ�ѧ��Ӧ����ʽ��
| ŨH2SO4 |
| �� |
| ŨH2SO4 |
| �� |
��1��ͨ�����Ϸ���֪��D�Ľṹ��ʽΪ��CH3COOCH2CH3��D�й�����������������
�ʴ�Ϊ��������CH3COOCH2CH3��
��2����Ӧ������ϩ��ˮ�����ӳɷ�Ӧ�����Ҵ�����Ӧ����ʽΪ��CH2=CH2+H2O
| һ�������� |
�ʴ�Ϊ��CH2=CH2+H2O
| һ�������� |
��3����Ӧ�����������Ҵ�����������Ӧ����������������Ӧ����ʽΪ��CH3COOH+CH3CH2OH
| ŨH2SO4 |
| �� |
�ʴ�Ϊ��CH3COOH+CH3CH2OH
| ŨH2SO4 |
| �� |

(8��)�״���һ�ֺܺõ�ȼ�ϣ���ҵ����CH4��H2O(g)Ϊԭ�ϣ�ͨ����ӦI��II���Ʊ��״���
��ش��������⣺
(1)��1.0molCH4��2.0molH2O(g)ͨ�뷴Ӧ��(�ݻ�Ϊl00L)����һ�������·�����Ӧ��
cCH4(g)+H2O(g) CO(g)+3H2(g) I��
CO(g)+3H2(g) I��
CH4��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��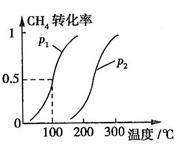
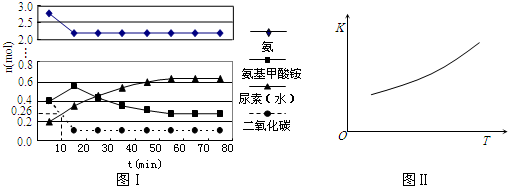
����֪100��ʱ�ﵽƽ�������ʱ��Ϊ5min������H2��ʾ��ƽ����Ӧ����Ϊ
��ͼ�е�P1 P2(�<������>����=��)��100��ʱƽ�ⳣ��Ϊ ��
�۸÷�Ӧ�� H 0��(�<������>����=��)
H 0��(�<������>����=��)
(2)��ѹǿΪ0.1MPa�����£�a molCO��3a mol H2�Ļ�������ڴ������������Է���Ӧ���ɼ״���CO(g)+2H2(g) CH30H(g)
CH30H(g)  H<0 ��
H<0 ��
���������ݻ����䣬���д�ʩ�����Ӽ״����ʵ��� (�����)��
| A�������¶� | B����CH3OH(g)����ϵ�з��� |
| C������He��ʹ��ϵ��ѹǿ���� | D���ٳ���lmolCO��3 mol H2 |
| ʵ���� | T(��) | n(CO)��n(H2) | p(MPa) |
| l | 150 | 1��3 | 0.1 |
| 2 | n | 1��3 | 5 |
| 3 | 350 | m | 5 |
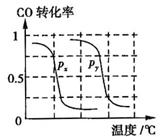
b�����ݷ�Ӧ����ص㣬��ͼ����ѹǿ�ֱ�Ϊ0.1MPa��5MPa��CO��ת�������¶ȱ仯������ͼ����ָ��ͼ�е�ѹǿ
 = MPa��
= MPa��
(8��)�״���һ�ֺܺõ�ȼ�ϣ���ҵ����CH4��H2O(g)Ϊԭ�ϣ�ͨ����ӦI��II���Ʊ��״���
��ش��������⣺
(1)��1.0molCH4��2.0molH2O(g)ͨ�뷴Ӧ��(�ݻ�Ϊl00L)����һ�������·�����Ӧ��
cCH4(g)+H2O(g)  CO(g)+3H2(g)
I��
CO(g)+3H2(g)
I��
CH4��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
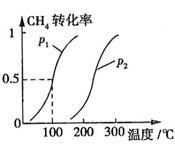
����֪100��ʱ�ﵽƽ�������ʱ��Ϊ5min������H2��ʾ��ƽ����Ӧ����Ϊ
��ͼ�е�P1 P2(�<������>����=��)��100��ʱƽ�ⳣ��Ϊ ��
�۸÷�Ӧ��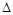 H
0��(�<������>����=��)
H
0��(�<������>����=��)
(2)��ѹǿΪ0.1MPa�����£�a molCO��3a mol H2�Ļ�������ڴ������������Է���Ӧ���ɼ״���CO(g)+2H2(g) CH30H(g)
CH30H(g) 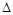 H<0 ��
H<0 ��
���������ݻ����䣬���д�ʩ�����Ӽ״����ʵ��� (�����)��
A�������¶� B����CH3OH(g)����ϵ�з���
C������He��ʹ��ϵ��ѹǿ���� D���ٳ���lmolCO��3 mol H2
��Ϊ��Ѱ�Һϳɼ״��������¶Ⱥ�ѹǿ��ijͬѧ���������ʵ�飬����ʵ�������Ѿ������������ʵ����Ʊ��С�
|
ʵ���� |
T(��) |
n(CO)��n(H2) |
p(MPa) |
|
l |
150 |
1��3 |
0.1 |
|
2 |
n |
1��3 |
5 |
|
3 |
350 |
m |
5 |
a���ϱ���ʣ���ʵ���������ݣ�n= ��m= ��
b�����ݷ�Ӧ����ص㣬��ͼ����ѹǿ�ֱ�Ϊ0.1MPa��5MPa��CO��ת�������¶ȱ仯������ͼ����ָ��ͼ�е�ѹǿ = MPa��
= MPa��






 ����д���֣�
����д���֣�